US-based Penumbra has completed enrolment for the THUNDER Investigational Device Exemption study of its advanced thrombectomy technology for acute ischemic stroke treatment.
This study will evaluate the safety and efficacy of the Penumbra System with Thunderbolt Aspiration Tubing, for blood clot removal in the brain.
The single-arm, multicentre trial targets patients with acute ischemic stroke caused by intracranial large vessel occlusion (LVO).
The primary efficacy endpoint of the study is the immediate post-procedure revascularisation of the occluded target vessel.
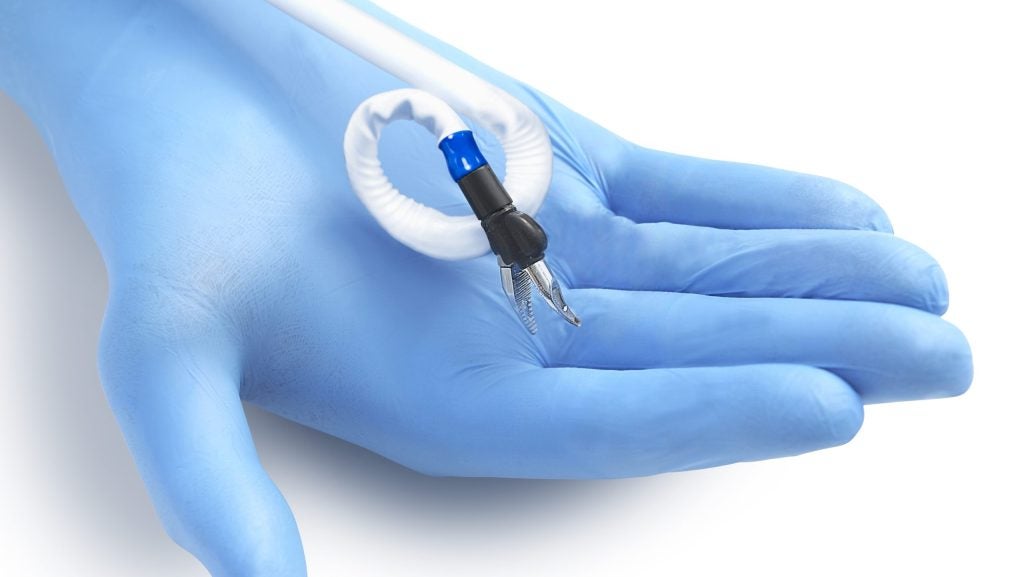
The Penumbra System with Thunderbolt Aspiration Tubing leverages a computer-assisted vacuum thrombectomy (CAVT) software algorithm.
This algorithm produces modulated aspiration, which minimises friction between the clot and the reperfusion catheter.
Consequently, it facilitates a swifter and more thorough removal of clots.
Thunderbolt Aspiration Tubing is specifically designed to be compatible with the Penumbra ENGINE and RED reperfusion catheters.
Penumbra president and CEO Adam Elsesser said: “This critical milestone brings us another step closer to providing physicians with the latest technology for stroke management.
“The THUNDER study will provide the data set needed to evaluate Penumbra’s Thunderbolt technology and I am optimistic that we are at the dawn of a new era in stroke treatment.”
In April this year, Penumbra secured US Food and Drug Administration approval and launched Lightning Flash 2.0, a next-generation CAVT system.
Featuring advanced algorithms for increased speed and sensitivity during clot removal, the Lightning Flash 2.0 system is intended for the removal of venous thrombus and treats pulmonary emboli.