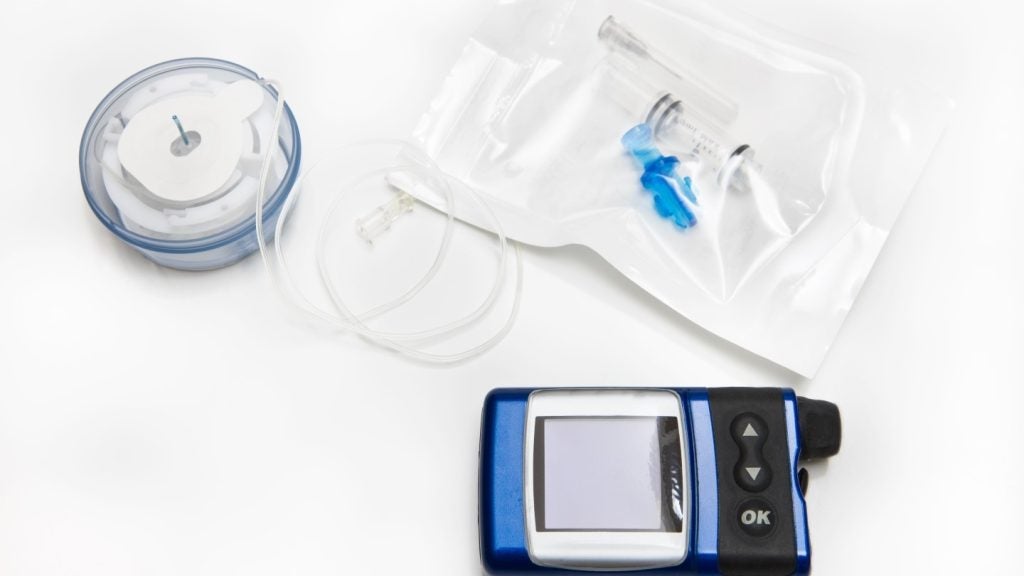
Switzerland-based medical devices maker PharmaSens has submitted an application to obtain approval from the US Food and Drug Administration (FDA) for its ‘niia essential’ insulin patch pump system.
For PharmaSens, the move is considered to be a significant milestone in advancing insulin pump technology.
It also follows the achievement of the ISO 13485 certification for the system in November 2023. It included the complete process involving the design, development, manufacture and distribution of insulin infusion pumps and accessories.
The company's product is claimed to have been designed as a patient-centric and user-friendly device.
First in a series of three patch pumps, the ‘niia essential’ system is known for its streamlined design and ease of use. It combines the simplicity of an insulin pen with the benefits of a sophisticated pump, addressing unmet needs in diabetes management.
The basal-bolus patch pump features a unique 3ml reservoir, offering an extended usage period and access to reimbursed therapy.
It is also claimed to be one of the most compact patch pumps available and incorporates an automatic needle insertion mechanism, ensuring the safety of the 90° steel needle.
PharmaSens's semi-reusable concept of the insulin pump system aligns with cost-efficiency and sustainable design, meeting the demand for environmentally friendly products.
PharmaSens CEO Marcel Both said: “Our submission to the FDA is a pivotal step towards making advanced diabetes care accessible. We are committed to transforming the experience of diabetes management through our innovative technology.
“As we anticipate a favourable review from the FDA, PharmaSens is gearing up for the market launch of our 'niia essential' insulin pump system. Recognising the importance of a strong partnership for successful market entry, PharmaSens is engaging in trade sale discussions."
Both added: “The current interest in PharmaSens suggests a perfect timing. Such a move would enable the new owner to focus on a market entry strategy with our scalable and soon-to-be-approved product.”