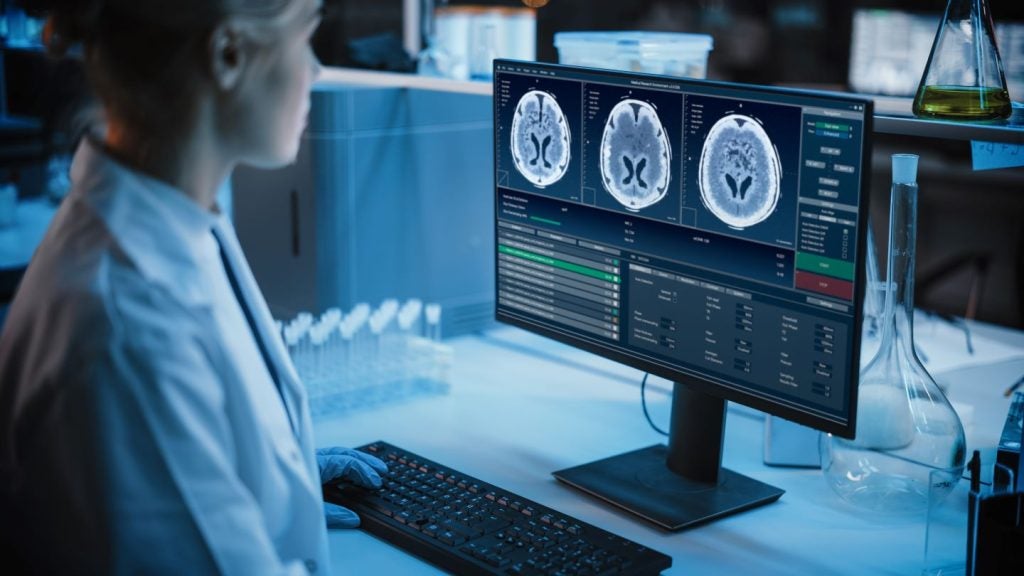
Pixyl has obtained 510(k) clearance from the US Food and Drug Administration (FDA) for its next-generation artificial intelligence (AI)-powered brain MRI analysis software, Pixyl.Neuro.
The new software is intended for analysing brain MRI images automatically to identify, diagnose and monitor neurological disorders rapidly.
The company has developed the software to enhance the detection of neurological disease activity, speed up MRI reading time and offer peace of mind for 83% of stable MS MRI exams.
Pixyl.Neuro helps quantify and compare brain region volumes to normative data for early abnormal atrophy detection and enables differential diagnosis.
It utilises minimal MRI protocols and delivers results within minutes.
Pixyl CEO Senan Doyle said: “It is incredibly rewarding to receive feedback from Pixyl.Neuro users who attest to this valuable support.
“We are delighted to work with US radiologists and imaging centres to reinforce radiology workflows and patient care.”
The company has already received the CE mark IIa certification for the Pixyl.Neuro software in the European Union under the new Medical Device Regulations.
Furthermore, this software is utilised in over 12 countries spanning North America, Europe and Africa.
UC Davis Neuroradiology Division chief Lotfi Hacein-Bey said: “Pixyl's FDA approval holds great promise for supporting the management of neurodegenerative and neuroinflammatory disorders.
“We chose Pixyl to answer our routine practice needs based on their track record of delivering high-quality brain MRI solutions.”