US-based medical devices company Procyrion has successfully completed a Series E funding round, securing $57.7m.
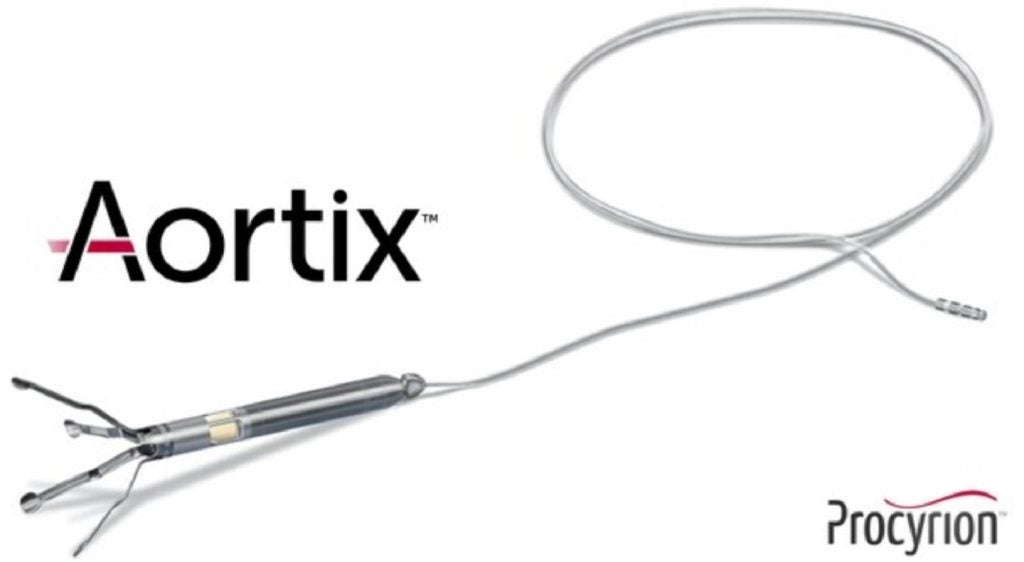
This investment will support the pivotal DRAIN-HF IDE trial of the Aortix pMCS device, designed to aid patients with acute decompensated heart failure (ADHF) who are not responding to standard medical therapy.
Led by Fannin Partners, the funding round saw contributions from both new and existing family and multi-family office investors.
Significant backing also came from repeat investors, including Bluebird Ventures and an undisclosed strategic investor.
Procyrion expects the capital to not only support the ongoing clinical trial but also enhance product manufacturability and facilitate commercialisation efforts.
Patients with cardiorenal syndrome (CRS) often struggle with standard intravenous diuretic therapy, leading to a harmful cycle where heart failure reduces kidney blood flow, impairing fluid removal and further burdening the heart.
Current treatment options are limited, as highlighted by the high rates of rehospitalisation or mortality within 30 days for patients discharged while still clinically congested, according to Procyrion.
Inserted via catheter into the descending thoracic aorta, the Aortix device aims to improve kidney perfusion and cardiac function.
Its unique design utilises fluid entrainment to pump blood without valves, offering a natural therapeutic action.
Results from the Aortix CRS pilot study showed patients experienced significant decongestion, haemodynamic improvement, and better kidney and cardiac function, with benefits lasting up to 30 days post-treatment.
Procyrion president and CEO Eric S Fain said: "Approximately 25% of the millions of patients admitted to the hospital with ADHF are unable to be successfully treated with standard of care therapies, yet there is a lack of effective treatment options available, leading to very poor outcomes.
“Aortix therapy is uniquely suited for treating CRS patients and this latest round of investment will enable the company to make significant progress toward commercialisation of our technology.”