German cardiovascular medical device company Protembis has secured US Food and Drug Administration (FDA) approval for its PROTEMBO Pivotal IDE Trial (NCT05873816).
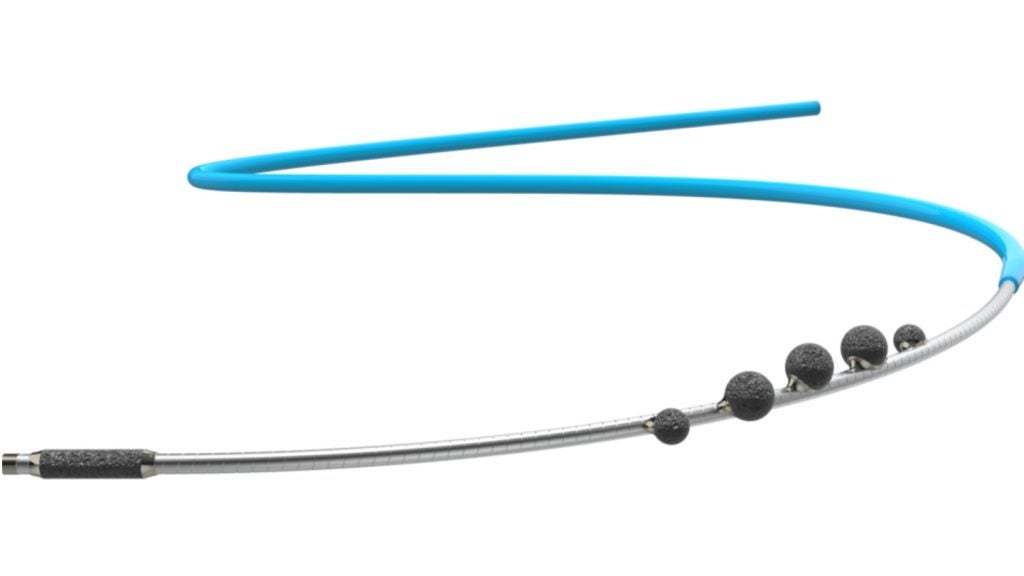
The ProtEmbo System from Protembis, is an intra-aortic filter device offering protection for the brain from embolic material released during a transcatheter aortic valve replacement (TAVR) procedure. It guards all cerebral vessels and allows clinicians to avoid interference with TAVR equipment during the procedure.
The trial will be conducted between a group of randomised patients undergoing TAVR in the US and Europe, ranging from 250-500 applicants. The aim of the trial is to demonstrate the superiority of ProtEmbo’s complete 3-vessel cerebral artery protection device against a hybrid control group.
Half of the group will receive no cerebral embolic protection (CEP), while the other half will receive the Sentinel CEP from Boston Scientific.
Following the FDA approval, Chair of the Study Executive Committee, Dr Roxana Mehran said: “We are excited to embark on this landmark trial as it is the only randomized trial in the space that is designed to examine the superior effectiveness of a next generation CEP technology to the current standard of care.”
The DW-MRI efficacy endpoint will use an adaptive statistical approach including pre-specified interim analyses with the possibility of early termination in the instance of superiority. The primary safety endpoint is the rate of major adverse cardiac and cerebrovascular events (MACCE) assessed 30-days in, with stroke neurologists adjudicating the neurological events.
Karl von Mangoldt and Conrad Rasmus Co-CEOs of Protembis expressed a joint message: “The Protembis team is working hard with our investigational sites, which are high volume TAVR centres and world-renowned academic centres of excellence, to ensure study activation is achieved expeditiously. The significant progress made reflects the close collaboration between our Global Steering Committee, our clinical research organization, and core lab partners in planning this complex trial.”