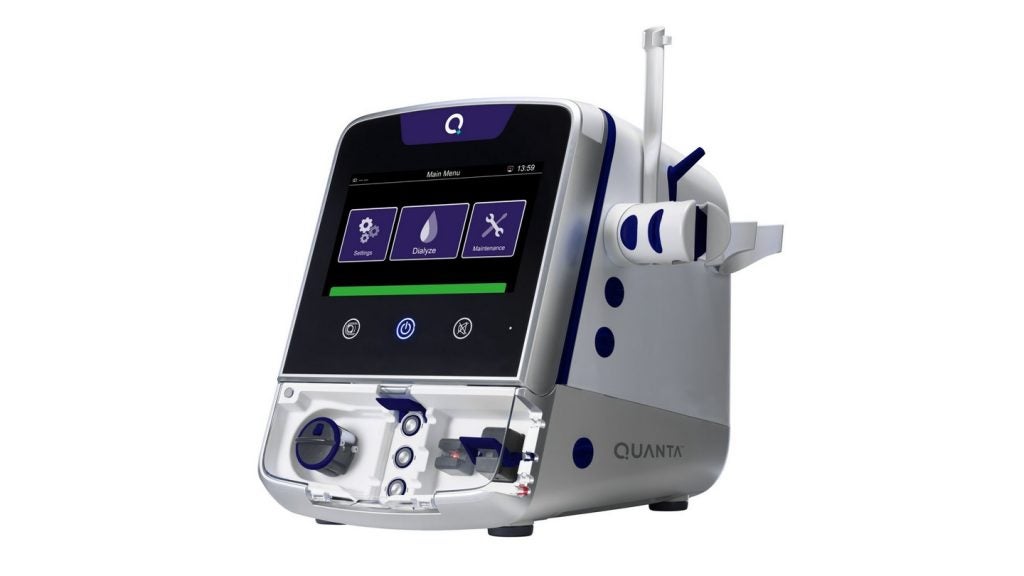
Quanta Dialysis Technologies has secured 510(k) clearance from the US Food and Drug Administration (FDA) for an expanded indication of its haemodialysis device, the Quanta Dialysis System.
The new indication has been approved for two modalities of continuous renal replacement therapy (CRRT), slow continuous ultrafiltration (SCUF) and continuous venovenous haemodialysis.
It is claimed to be the only dialysis device approved by the FDA for delivering intermittent haemodialysis, sustained low-efficiency dialysis or bagless CRRT through a single machine.
The expanded indication enables the use of the system for the treatment of critically ill patients diagnosed with acute kidney injury (AKI) who need dialysis.
The device offers flow rates ranging from 50ml/min to 500ml/min, along with the capability for continuous delivery for treatment times of up to 24 hours.
According to the company, the Quanta Dialysis System was originally created to address the needs of more than two million people globally who suffer from end-stage kidney disease.
Quanta is prepared to bring the Quanta Dialysis System featuring Trinal Kidney Therapy (TKT) software to the market and anticipates an official introduction of the product during the 2023 American Society of Nephrology Annual Meeting.
Quanta CEO Alejandro Galindo said: “Hospitals are often constrained with limited space and nursing staff.
“The Quanta Dialysis System with TKT software provides an all-in-one solution for hospitals with an intensive care unit looking to reduce their device footprint, maximise their operational efficiencies, reduce the burden on nurses and substantially lower consumables expenses.”