Realeve has secured a grant to provide its Pulsante micro-neuromodulation system for a study aimed at advancing post-stroke recovery.
The study will be conducted at the Cleveland Clinic. It will use the Pulsante system along with hyperbaric oxygen to potentially improve blood circulation in stroke patients, lowering brain damage and optimising neurological recovery.
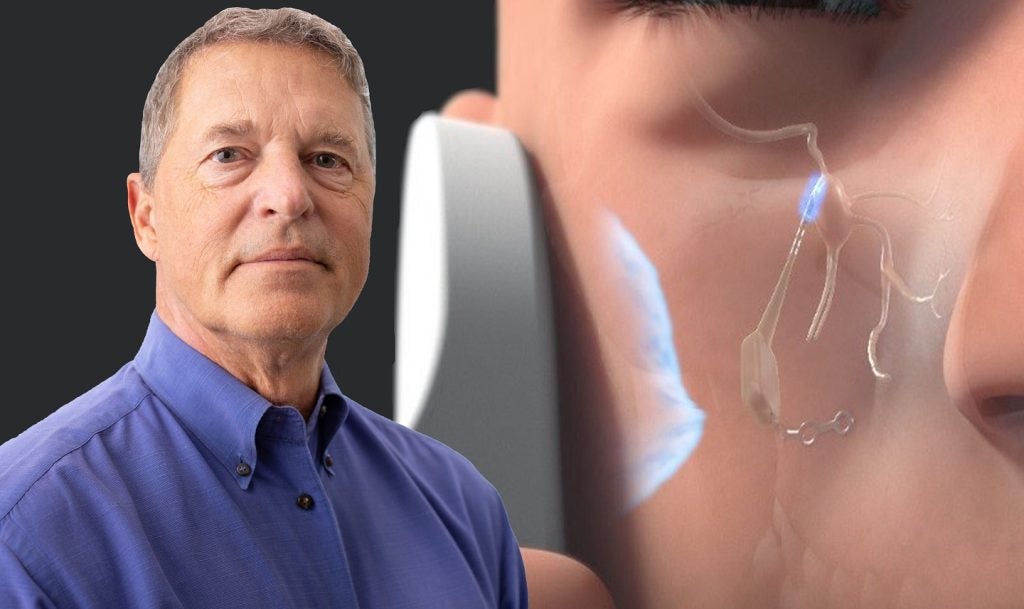
Pulsante is claimed to be the first externally powered implantable neuromodulation device that stimulates the sphenopalatine ganglion (SPG).
The device received a breakthrough device designation from the US Food and Drug Administration (FDA). It is expected to secure both FDA approval and a CE mark next year to treat cluster headaches.
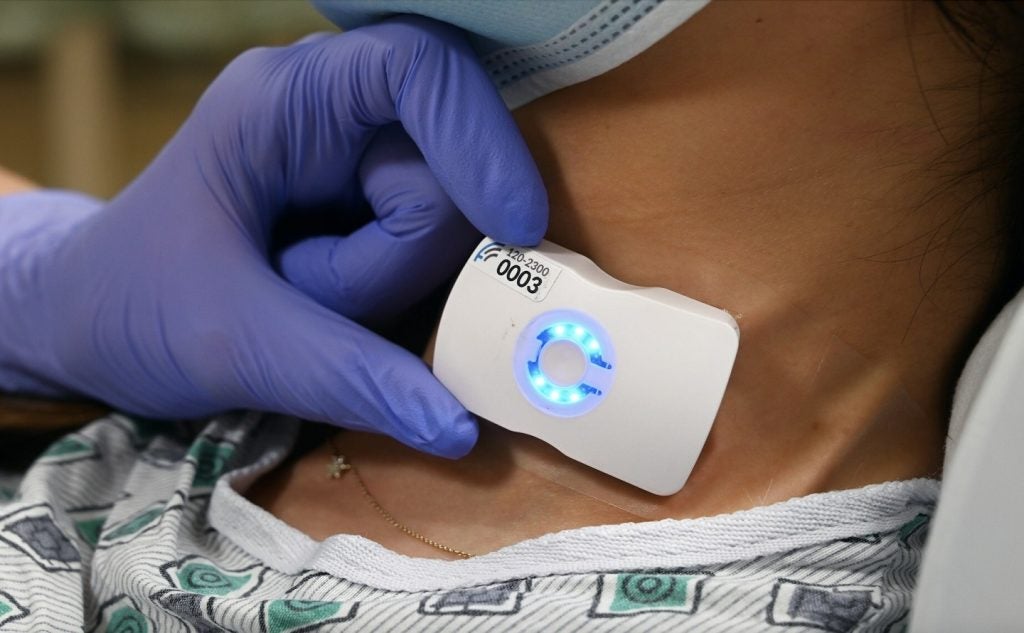
Pulsante features a miniature bioactive implant that is inserted orally, leveraging a minimally invasive technique that leaves no visible scars.
The device integrates with an external, hand-held controller that stimulates the SPG, which controls the autonomic nervous system.
The targeted therapy offers pain relief and can also enhance blood flow and medication delivery to the central nervous system (CNS).
Leveraging Pulsante technology, Realeve is assessing the potential effects of its SPG therapy on the blood brain barrier (BBB).
The BBB acts as a vascular gatekeeper to the CNS, controlling the entry of drugs and therapeutic treatments into the brain.
This technology holds the potential for addressing conditions such as tumours, Alzheimer's and other CNS disorders.
Realeve founder and president Dr Peter Bonutti said: “We are excited to leverage the power of this external implant that has been clinically proven with 700+ patients in enhancing post-stroke and cluster headache recovery.
“We are also exploring its applications in other central nervous system disease conditions and excited about the potential.”