Ricoh USA and Kallisio have entered a strategic partnership to produce and distribute the patient-specific oral stent, Stentra, which has been designed to advance radiation therapy for patients with head and neck cancer.
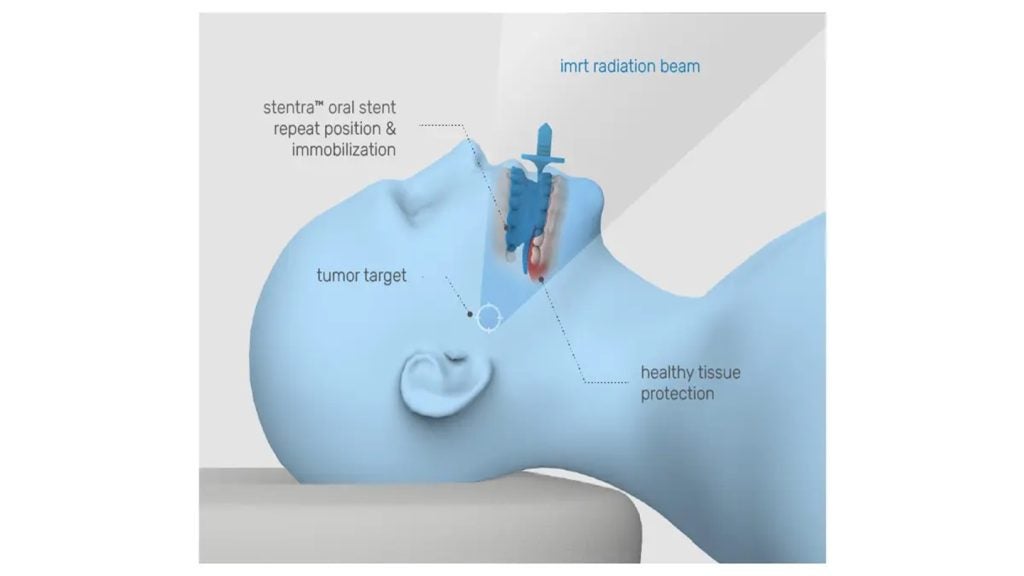
Stentra received 510(k) clearance from the US Food and Drug Administration (FDA) and is intended to protect healthy tissue during treatment.
Developed with technology exclusively licensed from MD Anderson Cancer Center, the stent is designed to precisely displace and immobilise sensitive areas, facilitating more targeted radiation delivery.
This is expected to help reduce side effects and improve treatment outcomes for patients.
Ricoh 3D for Healthcare will harness its network of 3D printing facilities registered by the FDA to fabricate Stentra.
Ricoh 3D Healthcare managing director Gary Turner said: “Stentra not only protects the tongue and other healthy tissues from exposure to radiation but also enhances the precision of radiation therapy, thereby reducing side effects and improving patient quality of life.”
As of next month, Stentra can be ordered by radiation oncologists through Ricoh 3D for Healthcare's clinical applications specialists.
Kallisio CEO Rajan Patel said: “Partnering with Ricoh 3D for Healthcare is a natural fit for our mission to transform cancer care. Ricoh's expertise in advanced manufacturing and its robust distribution network, including point-of-care production capabilities and clinical applications team, make them an ideal partner.
“Together, we can ensure that Stentra reaches radiation oncology teams efficiently, enhancing treatment precision and patient outcomes on a broad scale.”
Ricoh 3D for Healthcare and Kallisio will present Stentra at the ASTRO 2024 Annual Meeting, which will take place at the Walter E Washington Convention Center in Washington, DC, from 29 September to 2 October.
In June 2022, the FDA granted 510(k) clearance for Ricoh 3D for Healthcare's craniomaxillofacial and orthopaedic anatomic modelling.