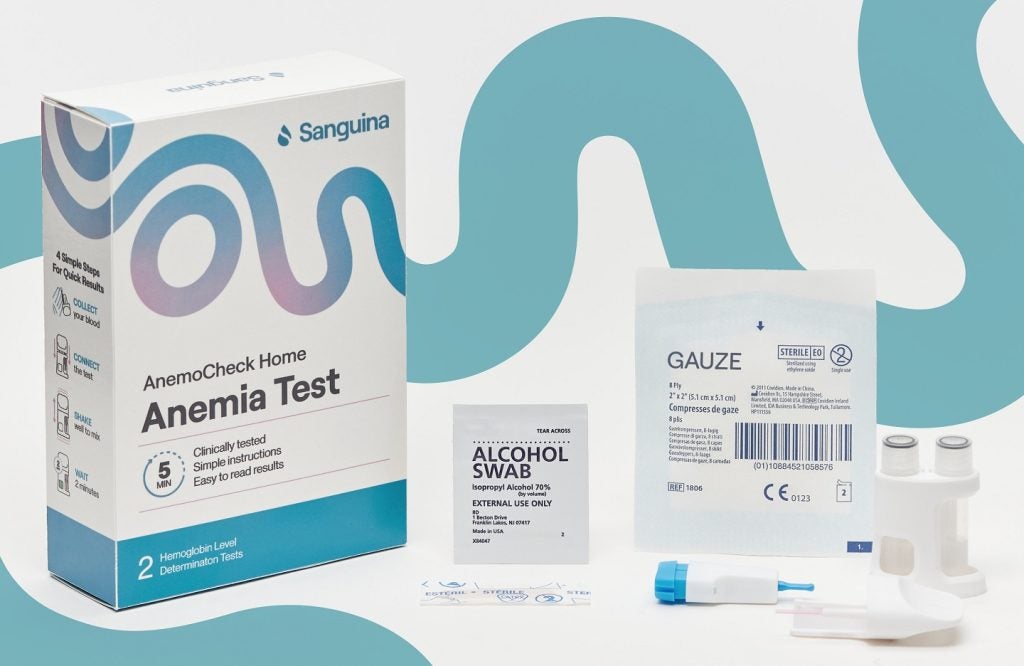
Sanguina has received clearance from the US Food and Drug Administration (FDA) for its haemoglobin test kit, AnemoCheck Home.
Said to be the only FDA-approved home haemoglobin test kit in the US, the test enables individuals with anaemia due to nutritional deficiency, thalassemia and sickle cell disease to monitor their haemoglobin levels from their homes.
The nutritional deficiencies include vitamin B12, folate and iron deficiency anaemia.
AnemoCheck Home, which is an in vitro diagnostic device, will be available only through prescription.
Sanguina’s disposable test helps users receive precise haemoglobin level readings by carrying out a simple fingerstick blood test.
The blood is collected into a tube and then the test cap is connected to a test body. Later, the user shakes the test to mix the blood.
The colour on the card correlates to the haemoglobin level after two minutes.
Sanguina CEO Erika Tyburski said: “Our team at Sanguina is proud to introduce AnemoCheck Home as a game-changer for at-home anaemia testing.
“With this FDA clearance, we are excited to provide people who have anaemia with a convenient, accurate and accessible tool to monitor their haemoglobin levels at home.”
Anaemia, a condition resulting from a deficiency of healthy red blood cells or haemoglobin, impacts more than 1.92 billion people globally.
Sanguina is focused on the development of digital and at-home health platforms for quick home-based testing and disease management.