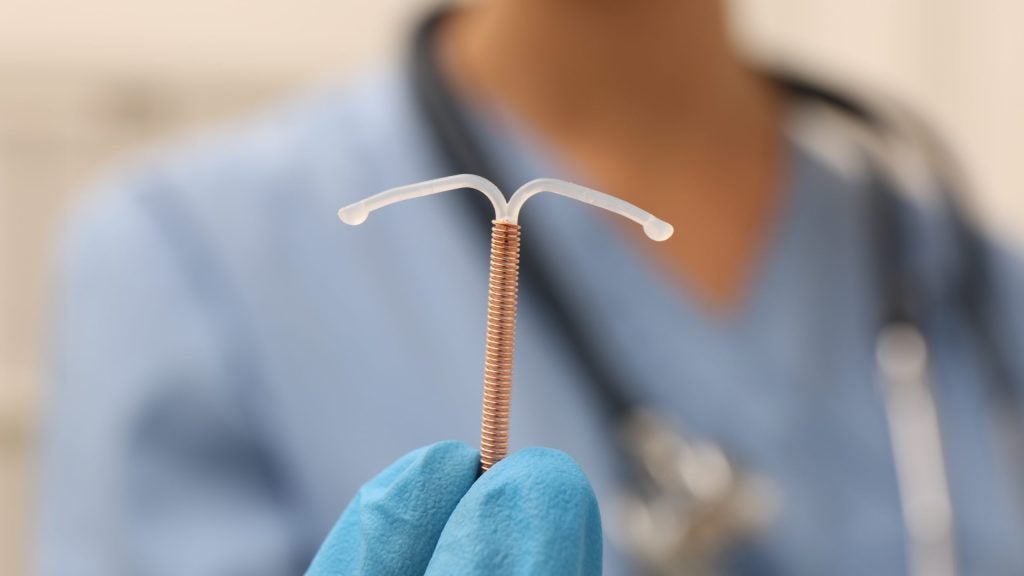
Sebela Women's Health has announced it has further positive results in a pivotal Phase III open-label study of the investigational copper, hormone-free intra-uterine device (IUD).
The data provides further support for the investigational, next-generation, hormone-free copper IUD, known as VeraCept, as exhibited in its pivotal phase III trial (NCT03633799), which met its primary endpoint of three-year contraceptive efficacy.
Sebela Women's Health head of research and development Kelly Culwell said: "This innovative investigational low-dose copper IUD clearly helps to fill a gap in the need for more than one hormone-free, long-acting, highly effective contraceptive option for women."
VeraCept is a copper-based, hormone-free, long-acting reversible intrauterine contraceptive device intended to prevent pregnancy. It is designed to deliver low doses of natural copper into the uterus.
According to GlobalData’s Medical Device Intelligence Centre, VeraCept is safe and effective in preventing pregnancies using less than half the amount of copper applied in the only other copper IUD on the market.
GlobalData is the parent company of Medical Device Network.
The estimated approval date for the Class III medical device according to GlobalData is August 2025 and the estimated launch date is November 2025. The global market for reproductive health devices is forecast to approach sales of $3.7bn by 2030.
Most recent data shows reduction in pain and bleeding
The post-hoc analysis of the Phase III study found that heavy menstrual bleeding among participants decreased over the three years, from 69% to 4.7%. During the same timeframe, dysmenorrhea reports decreased from 63.8% to 5.2%.
The primary outcome of the study was contraceptive efficacy through three years of use, as assessed by the Pearl Index. The most recent data reaffirmed the positive results of the pivotal Phase III study demonstrating the efficacy of the IUD with a cumulative three-year pearl index of 0.96 (95% CI, 0.59-1.48) or 99% efficacy. The study remains ongoing to assess use beyond three years.
The most common adverse events were similar to those seen with the use of IUDs – heavy menstrual bleeding, dysmenorrhea, and intermenstrual bleeding, with rates decreasing over time.