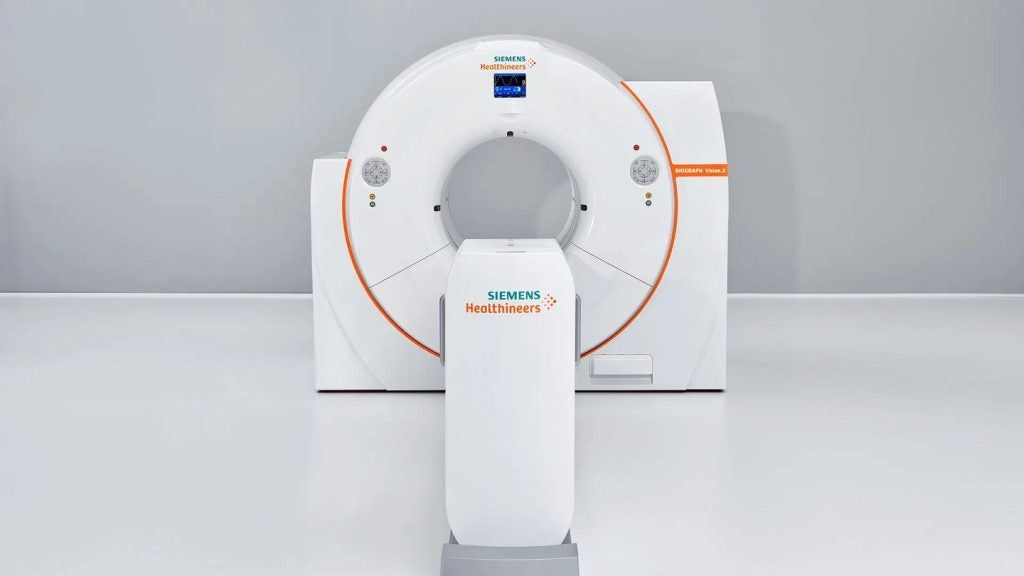
Siemens Healthineers has secured approval from the US Food and Drug Administration (FDA) for its positron emission tomography/computed tomography (PET/CT) scanner, Biograph Vision.X.
With a time of flight (TOF) of 178 picoseconds (ps), the Biograph Vision.X system features Optiso Ultra Dynamic Range (UDR) detector technology.
The UDR technology comprises silicon photomultipliers that allow the use of small 3.2mm x 3.2mm lutetium oxyorthosilicate crystal elements to provide higher spatial resolution than larger crystals.
Siemens said that the Biograph Vision.X is capable of delivering a high 48mm³ volumetric resolution and a temporal resolution of 178ps.
This allows the system to provide a 20% performance improvement (TOF gain) to lower patient radiation exposure and radiotracer costs while improving patient throughput.
Siemens Healthineers Molecular Imaging head James Williams said: “The Biograph Vision.X’s record-shattering, ultrafast time of flight delivers an image resolution that allows even the smallest lesions to rise above the noise.
“This extremely high level of resolution can help physicians detect small lesions and provide patients with a precise diagnosis.”
The scanner, which also features a built-in AIDAN intelligent imaging platform, makes use of a large 78cm bore to help calm anxious patients and easier positioning of radiotherapy devices or bariatric patients.
The AIDAN platform uses AI to increase operational efficiency and expedite patient workflow.
Biograph Vision.X is designed to fit into any room that holds a PET/CT scanner from the Biograph Vision family.
Earlier this month, Siemens Healthineers announced its plans to cut as many as 300 jobs from its diagnostics and manufacturing division as part of restructuring efforts across the company.
The decision will impact employees at the Siemens Healthcare Diagnostics manufacturing facility in Flanders, New Jersey, US.