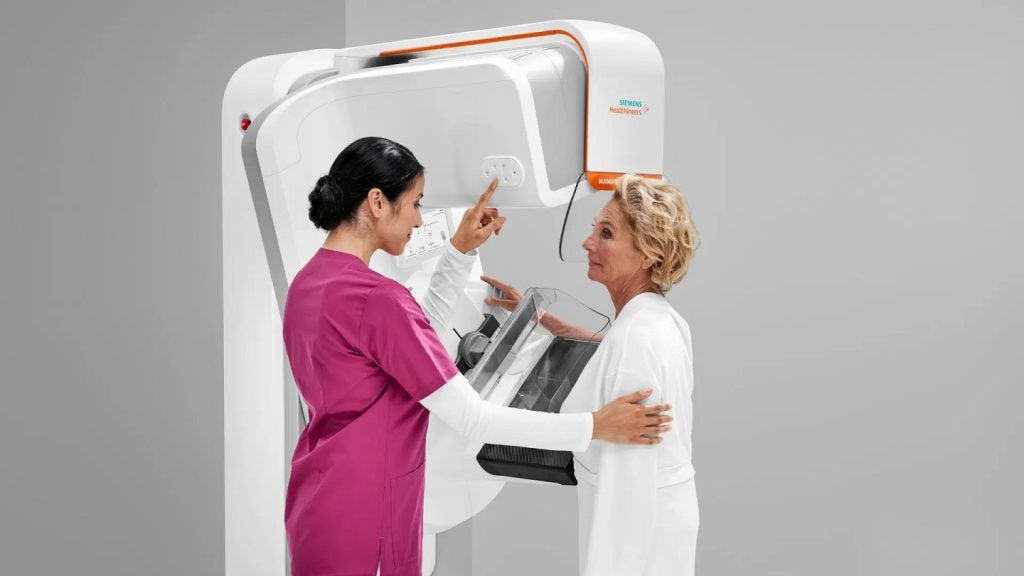
Siemens Healthineers has received 510(k) clearance from the US Food and Drug Administration (FDA) for its MAMMOMAT B.brilliant mammography system.
The system is a complete rebuild mammography platform and is designed to improve patient comfort and user ergonomics.
The clearance includes the system's 2D breast imaging, titanium contrast-enhanced mammography capabilities and breast biopsy.
Siemens Healthineers North America X-ray Products vice-president Niral Patel said: “With the 510(k) clearance of the MAMMOMAT B.brilliant, Siemens Healthineers proudly introduces completely new mammography technology to the market.
“We believe more women deserve access to next-generation screening technology, and this innovation underlies our commitment to women’s health.”
The MAMMOMAT B.brilliant system comprises several new features including a display monitor that allows clear visibility of patient information and workflow steps from any position.
It also features an automated ComfortMove ergonomic feature that minimises physical strain during patient positioning and a laser guide aids in accurate breast placement. These enhancements are expected to significantly improve workflow efficiency.
For patients, the MAMMOMAT B.brilliant offers the ComfortPackage, which includes an ergonomic hand grip, an optimised face shield for stability, and a personalised soft breast compression system.
Redesigned ambient light display and curved compression paddles further contribute to patient comfort.
The 3D tomosynthesis portion of the system, named PlatinumTomo, is under FDA review but is already available in Asia, Europe and South America. It has the widest angular range of 50° for tomosynthesis, matching the company's MAMMOMAT Revelation system.
Some of the features of the 3D technology include a detector and X-ray tube with z-Sharp technology, similar to that used in computed tomography scanners, allowing for continuous tube motion and stable focal spot size.
Last month, the FDA granted clearance for Siemens Healthineers’ CIARTIC Move, a self-driving mobile C-arm designed to automate repositioning during surgery.