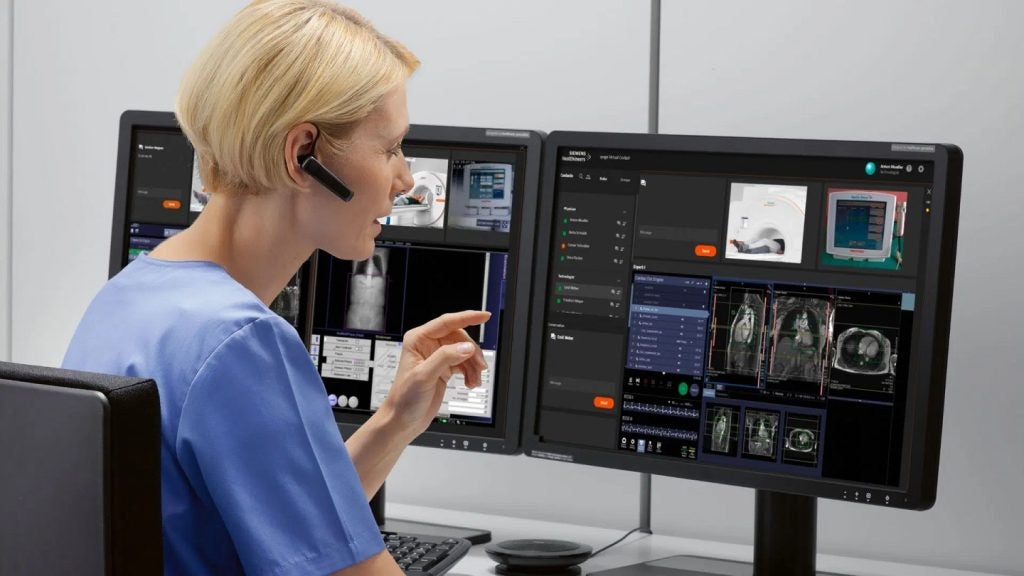
Siemens Healthineers has received US Food and Drug Administration (FDA) approval for syngo Virtual Cockpit, a software that facilitates real-time remote scanning and collaboration among healthcare professionals.
The multi-vendor scanning software allows radiologic technologists to connect with various scanners including magnetic resonance (MR), computed tomography (CT), positron emission tomography (PET), single-photon emission CT (SPECT), PET/CT, SPECT/CT and PET/MR from Siemens Healthineers and other equipment vendors.
It is designed to support private, secure communication and enables real-time image visualisation, acquisition and collaboration across multiple sites.
Siemens Healthineers North America digital and automation head Peter Shen said: “With this FDA clearance for syngo Virtual Cockpit, Siemens Healthineers proudly offers our customers the first and only multi-vendor, multi-modality, FDA-cleared solution for remote assistance scanning.
“This FDA clearance means that our customers can use syngo Virtual Cockpit for their remote scanning operations with even more confidence that they are using a proven solution – one that prioritises patient safety and convenience while also addressing operational and staffing challenges.”
The capabilities of the secure communication platform extend to allowing technologists to use live audio, video and chat functionalities for conducting scans or providing support for up to three different remote scanners simultaneously.
Furthermore, syngo Virtual Cockpit serves as a tool for on-the-job staff training at remote facilities.
By enhancing the local availability of advanced imaging, the software stands to benefit patients in suburban and rural areas who would otherwise face long travel distances for complex scans.
In November 2023, Siemens Healthineers secured FDA approval for its (PET/CT) scanner, Biograph Vision.X.