The US Food and Drug Administration (FDA) has granted 510(k) clearance for Techsomed’s ablation treatment planning and confirmation software, VisAble.IO.
Physicians can use the new software to plan liver ablation procedures and confirm ablation zones to increase treatment precision.
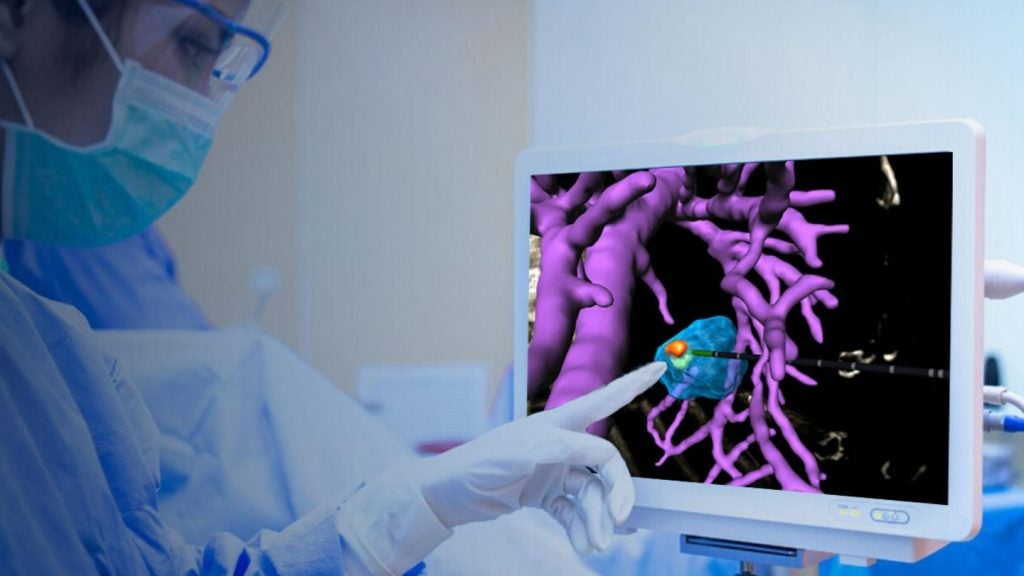
VisAble.IO marks the first release within the company’s full BioTrace solution, offering complete visualisation and control capabilities for image-guided ablation therapy.
BioTrace deploys artificial intelligence (AI) based technology to integrate common imaging methods, such as ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI).
This integration allows for real-time visualisation of the entire ablation zone, along with smart, personalised tools for treatment planning and assessment, facilitating optimised patient care.
Utilising advanced computation and image registration, the software helps physicians in key steps of planning and assessing the ablation coverage of a liver tumour.
The software offers various benefits, including 3D visualisation of the ablation target within a patient-specific anatomical view, overlaying virtual instruments and their positioning, and estimating ablation regions on medical images.
It also facilitates an interactive 3D view of ablation margins and missed volumes to help users evaluate ablation target coverage instantly after procedure.
Techsomed CEO and founder Yossi Abu said: “We are excited to receive 510(k) clearance for our VisAble.IO solution and appreciate the relentless efforts of our entire team in achieving this milestone.
“This is a pivotal step forward in our journey of elevating ablation therapy into image guided ablation therapy and making it available for every patient everywhere.”
The BioTrace platform also consists of the BioTrace.IO Lite software system, which is presently under assessment by the FDA via the De-Novo pathway.