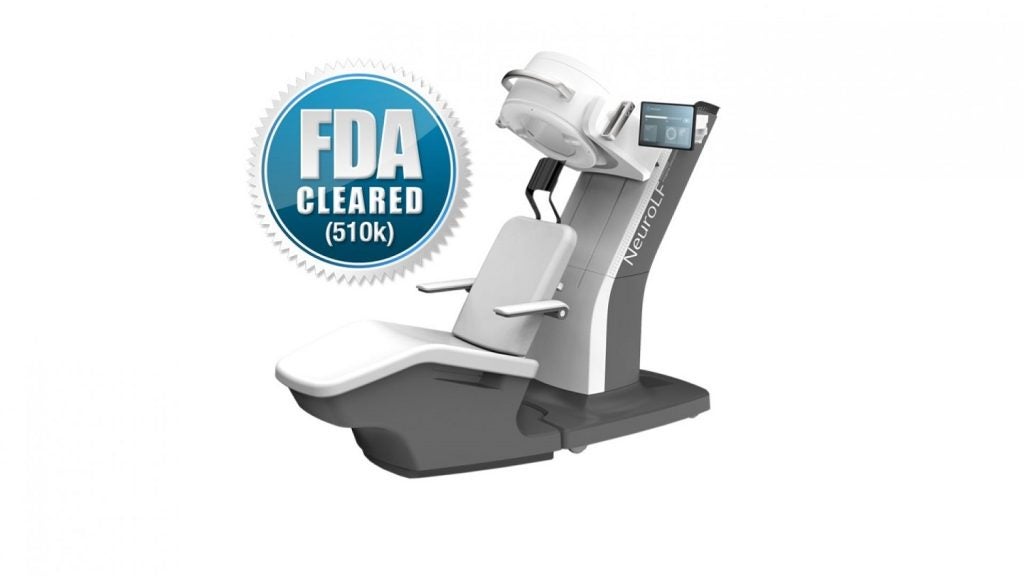
The US Food and Drug Administration (FDA) has approved Swiss company Positrigo’s NeuroLF brain positron emission tomography (PET) system.
The NeuroLF ultra-compact system is designed to aid in the diagnosis and monitoring of brain disorders such as Alzheimer's disease, epilepsy and Parkinson's disease.
This clearance marks a pivotal step for the company, enabling the introduction of the PET system to the US market.
NeuroLF represents a shift towards specialised medical imaging, allowing for detailed imaging of a particular body part or organ.
The system's compact design requires minimal space and no special room modifications.
It also offers the unique feature of allowing patients to be scanned in a seated position, facilitating functional imaging directly at the point of care.
The company anticipates regulatory approval for the device in Europe later this year, following the successful completion of the required Medical Device Regulation (MDR) audit.
With pre-orders already in place, Positrigo is preparing to scale up its production capabilities to meet the growing market needs.
Positrigo co-founder and CEO Dr Jannis Fischer said: “It is not the first device of its kind which receives market clearance in the US but we believe that our patient-centric and customer-driven design and development efforts over the last couple of years, brought us into the pole position to offer the best imaging solution to address the increased demand of brain PET scans.
“We are excited to fulfil numerous pre-orders in the US and to have the first customers benefiting from our technology very soon.”