West Pharmaceutical Services has received 510(k) clearance from the US Food and Drug Administration (FDA) for its Vial2Bag Advanced 13mm admixture device.
Designed for adolescent and adult patients, the new device has been introduced to complement West's existing Vial2Bag Advanced 20mm admixture device.
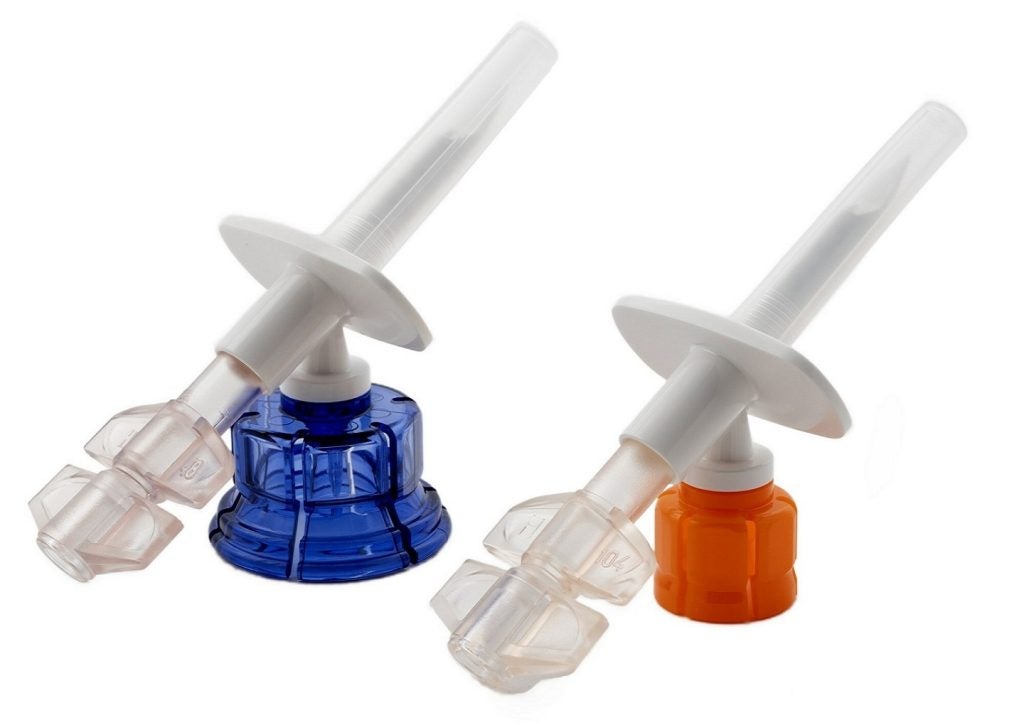
Both devices offer options for reconstituting and transferring drugs using either a 13mm or 20mm vial and an IV bag before administering to the patient.
They are intended for use only with a prescription.
The new needle-free device is designed to serve as a connection between a 50ml, 100ml or 250ml IV bag, a vial with a 13mm closure, and an external IV administration set.
With the integrated vial adapter, the reconstitution and admixing of drugs is possible before administration to the patient.
The Vial2Bag device features a dual-channel design for delivering fluid pathways into and out of the IV bag, as well as a vial spike design for connecting to the drug vial.
West Pharmaceutical Services chief commercial officer Cindy Reiss-Clark said: “This year, West celebrates 100 years as an industry leader and scientific innovator in high-quality injectable solutions.
“The 13mm product is a key addition to our administration system portfolio, addressing the critical need for more drug preparation and delivery options at the point-of-care.”
The company provides injectable solutions and services to its customers.
With 10,000 members across 50 sites worldwide, it annually delivers approximately 47 billion components and devices to support customers.
West reported $2.89bn in net sales during the fiscal year 2022.