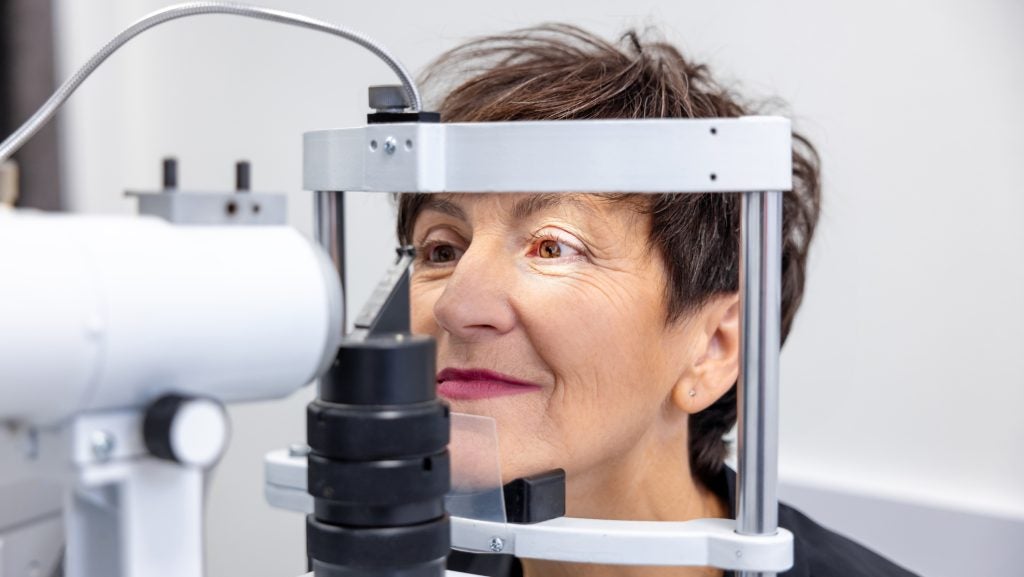
Germany-based device company ZEISS medical technology has received approval from the US Food and Drug Administration (FDA) for its laser system used in small incision lenticule extraction (SMILE) procedures to treat patients with near-sightedness.
VISUMAX 800 is equipped with ZEISS’ SMILE pro software, generating the lenticule in under ten seconds due to a rapid laser pulse repetition rate of 2MHz.
The SMILE pro software is a specialist programme designed by ZEISS that includes features and tools that assist in planning and executing the surgery, including aids for alignment, adjustments for potential eye movements during the procedure, and user nomograms for collecting and analysing patient data.
In the announcement accompanying the approval, Euan Thomson, the president of ophthalmology strategic business for ZEISS medical technology said: "As part of the ZEISS Medical Ecosystem, this next generation femtosecond laser system creates data-driven insights to help surgeons manage better treatment paths for patients while supporting each surgeon's unique practice requirements for greater workflow efficiency and performance."
In December 2023 ZEISS acquired Dutch Ophthalmic Research Center to expand its ophthalmic device portfolio in a huge deal valued at approximately €985m ($1.07bn). The acquisition is expected to close in H1 FY 2024. The deal comes in a bid to expand the company’s portfolio of vitreoretinal surgical devices, giving ZEISS access to gain access to the EVA NEXTUM platform, a dual-function system and accessories for vitreoretinal, cataract, and combined procedures.
The company received FDA approval in April 2023 for its aspheric, monofocal and single-piece C-loop intraocular lens (IOL), the CT LUCIA 621P Monofocal IOL. The lens is part of the ZEISS cataract workflow solution portfolio, focused on providing optimised visual outcomes for cataract patients.
In October 2023, ZEISS teamed up with Boehringer Ingelheim for the development of predictive analytics for eye diseases, focusing on detecting markers of early stages of retinal diseases by utilising ZEISS’s cloud-connected devices and artificial intelligence (AI)-assisted analysis of extensive image data sets.
According to a market model on GlobalData’s Medical Intelligence Center, the ophthalmic lasers market in the US is forecast to reach $649.9m in 2030.