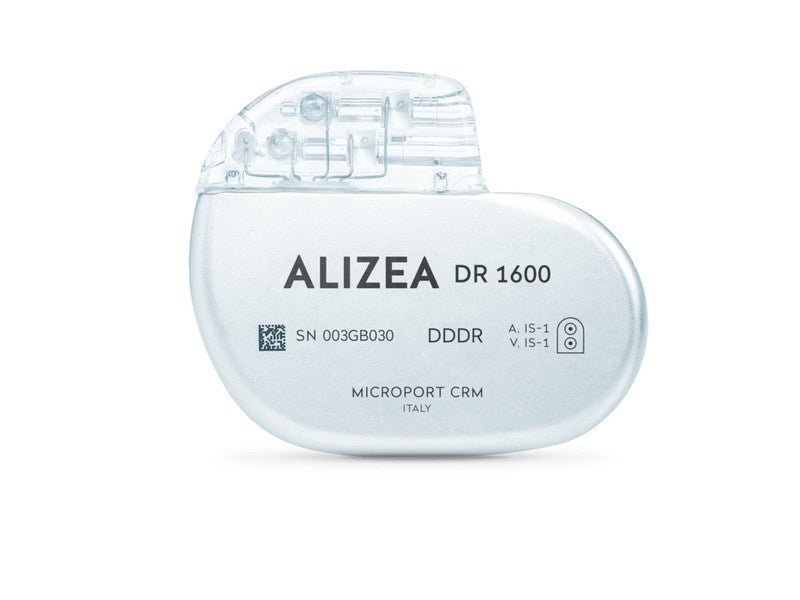
Alizea™ is an implantable cardiac pacemaker developed by France-based medical device company MicroPort® CRM for cardiac rhythm management in patients with cardiac rhythm disorders.
The pacemaker combines the latest technology, advanced functionality, and patient-centric design to provide superior therapeutic outcomes. It was launched in Europe in June 2021.
Alizea was launched in Japan in January 2022, followed by the US Food and Drug Administration (FDA) approval in May 2023. The FDA also approved the associated products, including the Vega™ pacing leads, SmartTouch XT™ tablet-based programmer, and SmartView Connect™ Bluetooth® home monitor.
Role of cardiac pacemakers
Cardiac pacemakers play a crucial role in the management of various cardiac rhythm disorders and in maintaining a regular heart rhythm, improving symptoms, enhancing the quality of life, and reducing the risk of serious complications associated with bradycardia, heart block, and certain types of arrhythmias.
The pacemakers are implanted under the skin in the patient’s chest area. They are connected to the heart by transvenous leads that conduct electrical impulses to pace the heart and restore a normal heart rhythm.
Alizea pacemaker system design and details
The Alizea is a small, compact and state-of-the-art pacemaker system designed for easier implantation and minimum visibility under the skin. The system consists of a pacemaker implant and a comprehensive monitoring platform, enabling seamless communication between healthcare providers and patients.
The device is implanted with the Vega pacing leads, which are rated for use in 1.5T and 3T MRI scanners.
SmartView Connect Bluetooth home monitor offers remote monitoring capabilities to the patients implanted with the pacemaker. The monitor, which can be placed next to the bed of the patient at home, automatically transmits detailed reports of the patient to the cardiologist at regular intervals. It can also transmit abnormal rhythms alerts such as atrial fibrillation, or alerts triggered by patients when experiencing symptoms. The monitor is designed with an easy-to-use interface for the elderly patient population.
The pacemaker system incorporates advanced sensing algorithms to accurately detect and classify various cardiac arrhythmias, enabling precise diagnosis and tailored treatment plans.
It features the AutoMRI mode, the advanced algorithm of MicroPort CRM, which improves patient safety and quality of life while undergoing an MRI examination. The patients visit their cardiologist once within ten days before their MRI scan to activate the MRI mode.
The pacemaker automatically switches to the MRI mode upon entering the MRI field once the mode is programmed. It reverts to its initial settings after the examination, significantly improving the workflow for both patients and medical staff.
The pacemaker has a projected longevity of 13 years at a volume of just 11cc, making it the longest-lasting pacemaker for its size currently available in the market.
Features of Alizea pacemaker system
The pacemaker communicates wirelessly with the monitoring platform using Bluetooth technology, to enable seamless transmission of patient data to healthcare professionals for evaluation and intervention.
Bluetooth-enabled remote monitoring facilitates early detection of device malfunctions, arrhythmia recurrences or other abnormalities, enabling healthcare providers to intervene promptly and prevent potential complications.
Greater longevity in pacemakers prevents patients from undergoing extra pacemaker generator changes and avoids the risk of related infection complications.
The SmartView Connect home monitor removes the need for patients to visit the hospital for simple routine examinations, reducing the burden on the healthcare system.
Alizea pacemakers and the SmartView Connect home monitor work together to provide the cardiologist with timely alerts and transmissions triggered by patients when they present symptoms, leading to faster and more efficient patient care.
The combination of the advanced features and technologies in the Alizea pacemaker systems ensures precise diagnosis, personalised treatment, enhanced patient safety, and improved quality of life for individuals with cardiac rhythm disorders.
SmartTouch XT tablet-based programmer
SmartTouch XT is a lightweight programmer tablet for mobile usage. Weighing 2.1kg, it has a modular design, which facilitates versatile adaptation in different clinical environments. It comes with an inductive programming head and dongle as well as a power cable and adapter.
The Bluetooth connectivity allows the implantable device and accessories to wirelessly communicate with the programmer.
Marketing commentary on MicroPort CRM
MicroPort CRM, a subsidiary of MicroPort Scientific, aims to bring the most advanced cardiac technologies to patients and physicians.
The company develops, manufactures and markets cardiac pacemakers, implantable cardiac defibrillators, cardiac resynchronisation systems, and electrocardiogram (ECG) diagnostic solutions in more than 50 countries, for the management of cardiac rhythm disorders and heart failure.
Its state-of-the-art products are manufactured in France, Italy, and the Dominican Republic.