Barostim Neo is a neuromodulation system that actively stimulates natural sensors in the human body that guide the brain to regulate the heart, to relieve the symptoms of heart failure.
Developed by CVRx, the system received a CE mark from the National Standards Authority of Ireland in September 2014, to treat heart failure patients with an ejection fraction less than or equal to 35%.
The system label was expanded as MR Conditional in December 2014, indicating that the device can be safely used in magnetic resonance imaging (MRI) systems under the provided conditions.
The system received breakthrough device designation from the US Food and Drug Administration (FDA) in June 2015. The FDA granted premarket approval (PMA) to the device in August 2019 for patients with advanced heart failure symptoms and ineligible for cardiac resynchronisation therapy (CRT).
Barostim Neo is approved for heart failure treatment in more than 30 countries and to treat resistant hypertension in Europe, Columbia and New Zealand.
In May 2022, CVRx received FDA approval for magnetic resonance conditional labelling for its Barostim System.
The Barostim System now incorporates guidelines enabling safe MRI scans of the head and lower extremities.
The advancement expands diagnostic possibilities for heart failure patients with Barostim implants. All patients utilising the Barostim system, including those currently undergoing Barostim therapy, are eligible for safe MRI scans at 1.5T, provided specific conditions are fulfilled.
Barostim Neo system details and features
Barostim Neo is a next-generation, minimally-invasive and implantable system developed using CVRx’s proprietary technology, Barostim Therapy.
Barostim is the only heart failure device therapy that does not require any hardware in the heart or blood vessels.
The system comprises an implantable pulse generator, a carotid sinus lead (CSL) kit, a wireless programmer system and a CSL repair kit.
The implantable pulse generator (IPG) measures 50mm wide, 72mm high and 14mm thick.
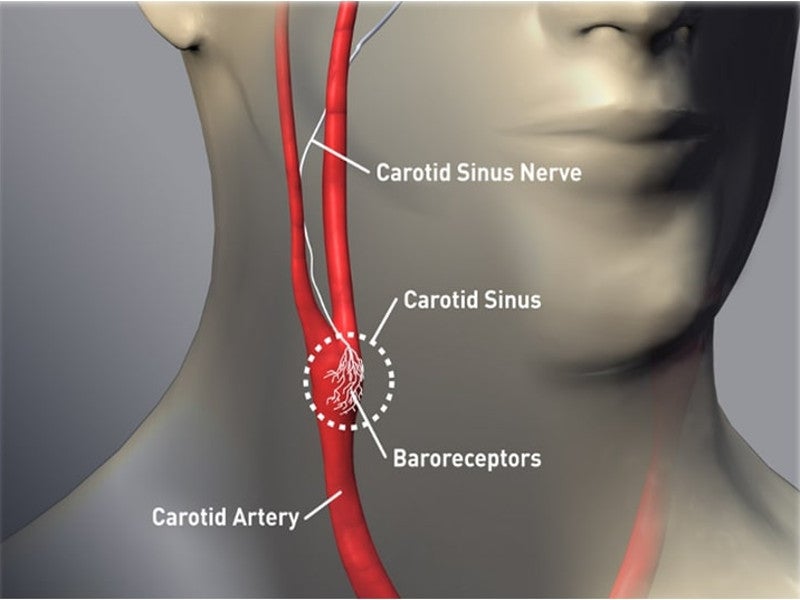
It encloses a battery and a circuit, which delivers activation energy to the baroreceptors placed on the right or left carotid artery.
The CSL kit contains a 2mm electrode and an implant tool interface, connected to the IPG through the connector module.
The wireless programmer system enables communication with the IPG. The therapy specifications are fed into the device while the IPG status information is collected by the programmer system.
The CSL repair kit contains the necessary materials required to repair the parts and accessories of the system.
Partnership between Hackensack University Medical Center and CVRx
In February 2020, Hackensack Meridian Hackensack University Medical Center became the first hospital in the US to implant the world’s first heart failure neuromodulation device, Barostim Neo.
The innovative technology is an effective alternative for patients with congestive heart failure (CHF), ineligible for other forms of therapy such as CRT.
The state-of-the-art device possesses a distinctive capability to enhance heart function, lower hospital readmission rates, and decrease patients’ medication needs. Additionally, it enhances patient mobility and lowers the likelihood of long-term infection as it lacks a pump or catheter, unlike alternative devices.
Hackensack University Medical Center partnered with CVRx in 2006 to participate in FDA-approved clinical trials to test the effectiveness and safety of carotid baroceptor stimulation to treat refractory hypertension.
The clinical trials revealed that the device was effective in treating CHF.
Hackensack University Medical Center participated in the feasibility trial Hope4HF and the pivotal trial Baroreflex Activation Therapy for Heart Failure Pivotal Trial.
The positive outcomes of these studies led to the FDA approval of Baroflex Activation Therapy for the treatment of patients with NY Heart Association Functional Class III CHF.
Barostim Neo system functioning details
The IPG is implanted subcutaneously below the collarbone and connected to the baroreceptors through the CSL. It sends electrical impulses to the carotid artery and activates the baroreflex, a natural mechanism of the body to maintain cardiovascular functioning.
The activation of the pathway lowers the sympathetic activity and increases parasympathetic activity to maintain the sympathovagal balance.
Clinical studies on Barostim Neo system
The FDA approval for the system comes from a two-phased, multicentre, randomised, controlled clinical trial, Baroreflex Activation Therapy for Heart Failure. A total of 408 patients across 92 sites were enrolled to study the safety and effectiveness of the device.
Major adverse neurological and cardiac events (MANCE) and event-free rates were determined at six months to evaluate the safety of the device. The effectiveness of the device was assessed by a six-minute hall walk (6MHW) based on the Minnesota Living with Heart Failure (QoL) questionnaire data and testing of N-terminal pro-brain natriuretic peptide (NT-proBNP), a prohormone related to cardiac disorder.
The first phase of the trial, the expedited phase, supported the PMA under the FDA’s Breakthrough Devices Program. Long-term information is being collected under the ongoing second phase, the extended phase.
Patients were randomised to receive either Barostim Therapy by Barostim Neo system along with medical management or medical management alone. At the end of six months, 96.8% of the patients with Barostim Neo system plus medical management remained free from MANCE.
The device was found safe for patients with heart failure symptoms, having reduced ejection fraction. Patients demonstrated improved quality of life by 14 points according to the QoL questionnaire.
The exercise capacity of the patients improved by 60m according to the 6MHW test while the functional status improved under New York Heart Association classification. The level of NT-proBNP in patients using the system also reduced significantly.