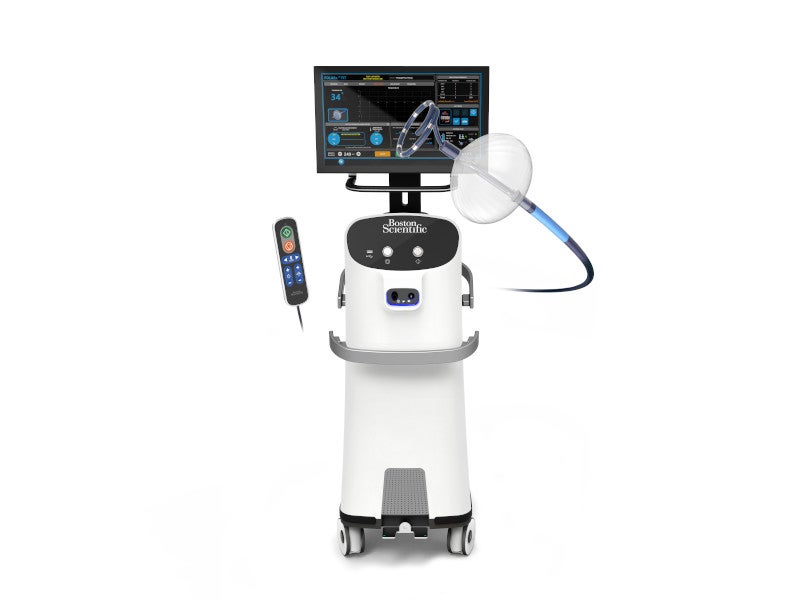
The POLARx is a new cryoablation system developed by Boston Scientific, a medical device company based in the US, for the treatment of paroxysmal atrial fibrillation (AF).
Cryoablation is a minimally invasive procedure to address AF, where a specialised balloon catheter is employed to administer cryotherapy to the pulmonary vein. The process involves freezing problematic tissue and forming scar tissue that impedes irregular electrical signals. The system received the European CE mark in February 2020 and approval from the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) in October 2021.
The POLARx cryoablation system with the POLARx FIT cryoablation balloon catheter received the US Food and Drug Administration (FDA) approval in August 2023. The POLARx FIT catheter was approved in Europe, Japan, Canada, and other Asia Pacific markets in 2023.
The system has been used by more than 25,000 patients worldwide, representing new cryoablation capabilities and advancements in the treatment of AF.
POLARx cryoablation system details
POLARx medical device system is designed to improve the cryoablation capabilities of AF. It includes four primary components: the POLARx FIT cryoablation balloon catheter, the SMARTFREEZE console, the POLARSHEATH steerable sheath, and the POLARMAP circular mapping catheter.
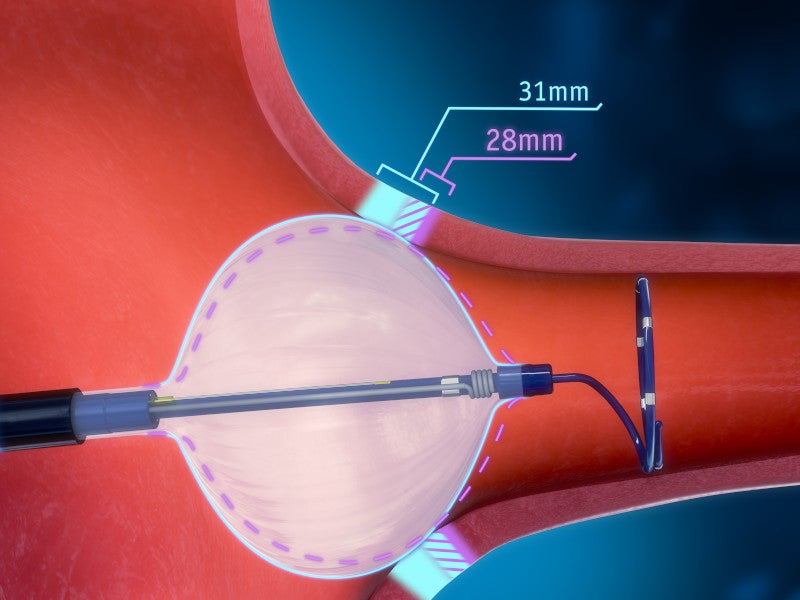
The POLARx FIT cryoablation balloon catheter is an innovative device with a dual-diameter balloon size in one catheter. The expandable design allows it to be adjusted to a 28mm balloon size, which can be expanded to 31mm. The 31mm balloon size increases the potential for full pulmonary vein occlusion, reducing the requirement for repositioning.
The innovative, semi-elastic, thermoplastic material of the catheter simplifies the delivery and placement of the balloon in the pulmonary vein with different shapes and anatomy. The manoeuvrability and variable balloon sizes make the system valuable for addressing challenges associated with varying cardiac anatomies. Furthermore, it introduces occlusion capabilities not typically seen with traditional systems. It offers 20% greater surface area and uniform pressure to occlude and treat different anatomies with more confidence.
The system maintains a stable 28mm balloon size during the inflation and therapy phases to prevent displacement during the balloon expansion.
SMARTFREEZE console details
SMARTFREEZE is an intuitive yet customisable console for the ablation procedure, which allows the implementation of several innovations in the procedure. It is designed to be more modern and customisable, with centralised key data viewing ability for increased control and an optimised workflow.
SMARTFREEZE software version 4.2 futher enhanced the existing technology to bring more innovations to the cryoablation procedure. Some of the features are a five-port interconnection box (ICB), a handheld remote control, Boston Scientific’s LABSYSTEM PRO to SMARTFREEZE conductivity, CIRCA Scientific S-Cath temperature probe, pulmonary vein occlusion pressure transducer and system workflow improvements.
POLARSHEATH steerable sheath
The POLARSHEATH steerable sheath improves accessibility in the pulmonary vein approach. The long, gradual taper, which runs from dilator to sheath with a tight bend radius, is designed for atraumatic entry at the femoral site and transseptal crossing location. Greater distal sheath deflection improves alignment capabilities for easier balloon positioning.
A 155º deflectable tip enables smooth access to the right inferior pulmonary vein and easier co-axial alignment in diverse anatomies.
POLARMAP circular mapping catheter
POLARMAP is a 20mm circular catheter with eight electrodes, which are 6mm apart. It is essential for optimised signals in an ablation procedure.
It features 5mm and 12mm tip lengths with insulated continuous core wire, insulated individual electrode signal wires and additional electrical insulation for reduced noise.
It improves the visualisation of pulmonary vein potentials by 14% during freeze.
POLARx cryoablation system features
The system eliminates the need for time-consuming and disruptive device changeouts while also enabling the treatment of a wider range of pulmonary vein anatomies.
It offers cryoballoon stability with enhanced sheath steerability and manoeuvrability. It has more effective console enhancements and mapping catheters.
Clinical trials on the POLARx cryoablation system
The effectiveness and safety of the POLAR cryoablation system were demonstrated in the FROZEN-AF IDE clinical trial. It is a global, prospective, non-randomised, single-arm clinical study involving 385 patients with paroxysmal AF.
The primary event-free rate, indicating freedom from procedure or device-related events, stood at 96% at 12 months. The patients achieved a 79.9% rate of freedom from documented atrial arrhythmias at 12 months.
Notably, no instances of moderate or severe pulmonary vein stenosis, persistent phrenic nerve palsy, or oesophageal fistulas were reported during the trial.