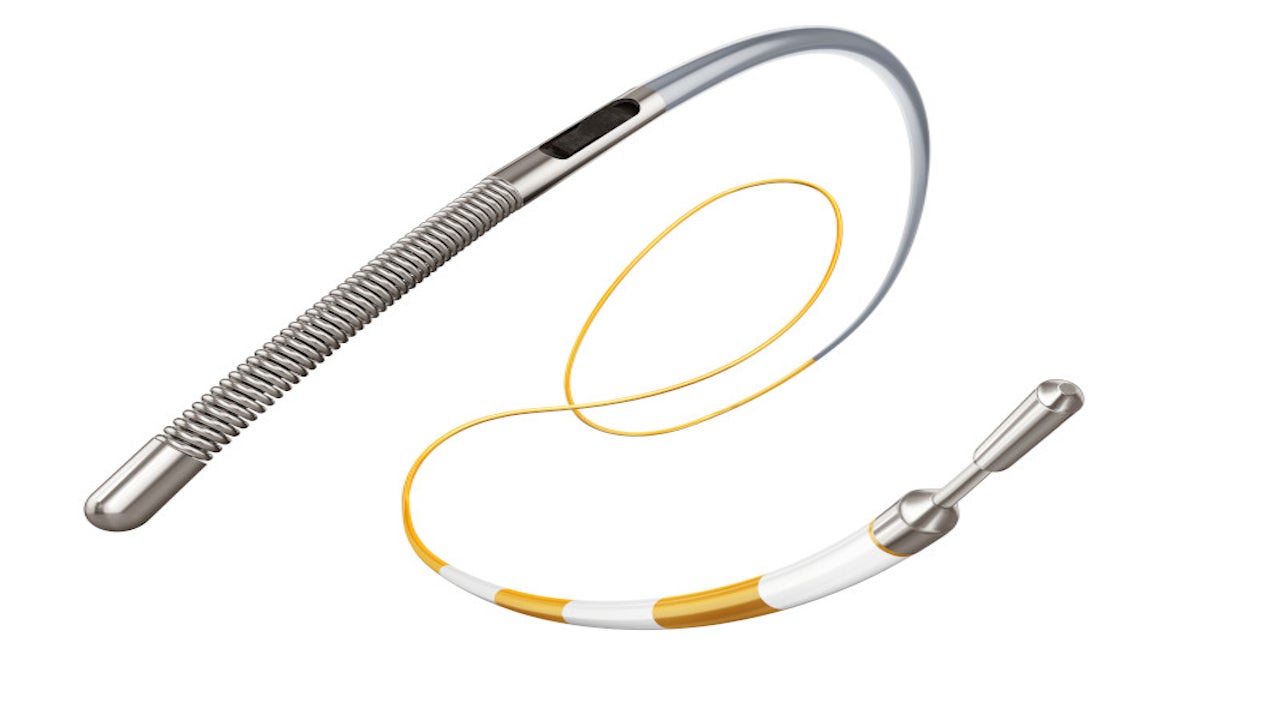
Philips’ OmniWire is the first solid core pressure guide wire available for coronary artery interventional procedures.
OmniWire is indicated for the measurement of pressure in blood vessels, including coronary and peripheral vessels during any diagnostic or therapeutic treatment, providing haemodynamic information to diagnose and treat blood vessel diseases.
The wire can also be utilised to place catheters or any other interventional devices in the vessels, commercially available in the US and Japan.
Details of OmniWire
OmniWire is a navigable guide wire equipped with a pressure sensor at 3cm proximal to the tip, available in two types of tip shape, a straight tip and a J-tip. The device has a diameter of 0.36mm, as well as flexible and working length of 40cm and 185cm, respectively.
The sterility assurance level (SAL) of the guide wire is 10-6. Its distal portion is made of Nitinol, a super-elastic, robust plastic that is widely found in non-diagnostic, interventional ‘workhorse’ guide wires. The portion has a hydrophilic coating to a length of 39cm to minimise surface friction and improve lubricity.
The proximal portion of the guide wire is made of a high-strength cobalt alloy with hydrophobic coating across 146cm length, offering the high reliability required for complicated and multi-vessel procedures.
OmniWire’s revolutionary solid core structure features embedded specialised conductive ribbons in its outer polymer layer to convey pressure information. This innovation makes its larger solid core feasible, similar to the workhorse guide wire design, offering the handling and reliability required for today’s dynamic and multi-vessel applications.
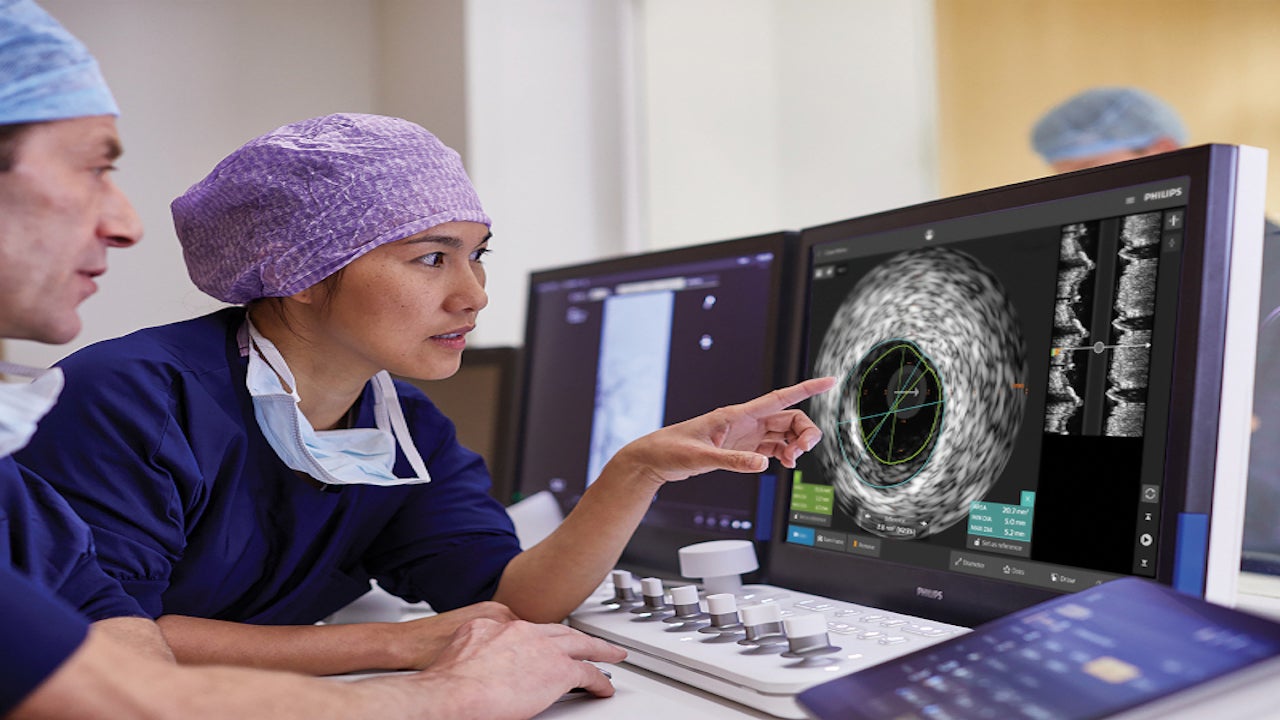
The guide wire incorporates the interventional applications platform Philips’ IntraSight, and SmartMap systems for pressure measurement during endovascular procedures.
OmniWire supports measurements of instant wave-free ratio (iFR), the only resting index backed by randomised regulated outcome experiments, as well as fractional flow reserve (FFR) measurements.
IFR is the leading hyperaemia-free physiological index for diagnostic and interventional pressure measurements. It is an evidence-based approach that enhances performance, saves time and reduces patient discomfort when compared to FFR.
IFR is implemented in clinical research and has been verified in the findings of clinical trials with more than 4,500 patients. It is also recognised by the European Society of Cardiology (ESC), the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC) and National Cardiovascular Data Registry (NCDR).
IntraSight features
IntraSight is integrated with a comprehensive suite of scientifically proven modalities including iFR, FFR, IVUS (intravascular ultrasound) and co-registration to simplify complicated treatments and accelerate routine procedures.
IFR pullback and co-registration enable physicians to locate the exact part of the vessel causing ischemia, plan stent size and positioning with the virtual stent, and forecast physiological recovery. OmniWire co-register iFR data onto the angiogram to accurately classify the areas of the vessels that need treatment.
IntraSight applications platform combines visualisation, pathology, co-registration and software to easily recognise coronary and peripheral artery disease, and facilitate better treatment strategies.
Philips’ IntraSight optimises lab efficiency with touchscreen control at tableside, systems integration, data management, and remote service diagnostics.
IntraSight improves user experience with an advanced, intuitive interface that reduces learning curves and enhances workflow confidence.
Benefits of OmniWire
The solid core design of the guide wire allows easier manoeuvrability of the wire in the circulatory system of the patient for blood pressure measurement along the vessel and direct catheter and stent delivery.
The solid proximal core of the guide wire is more like a workhorse wire. Embedded conductive ribbons remove the need for the traditional hollow hypotube, improving the torque response, durability, pushability and reducing the risk of kinking.
Nitinol distal core is extremely elastic for stability and structure recovery, helpful for prolonged multi-vessel procedures.
The integrated conductive bands improve signal reliability. The design of OmniWire has been thoroughly tested to provide a stable signal across 30 disconnections and reconnections while handling tortuous vessels as small as 1mm.