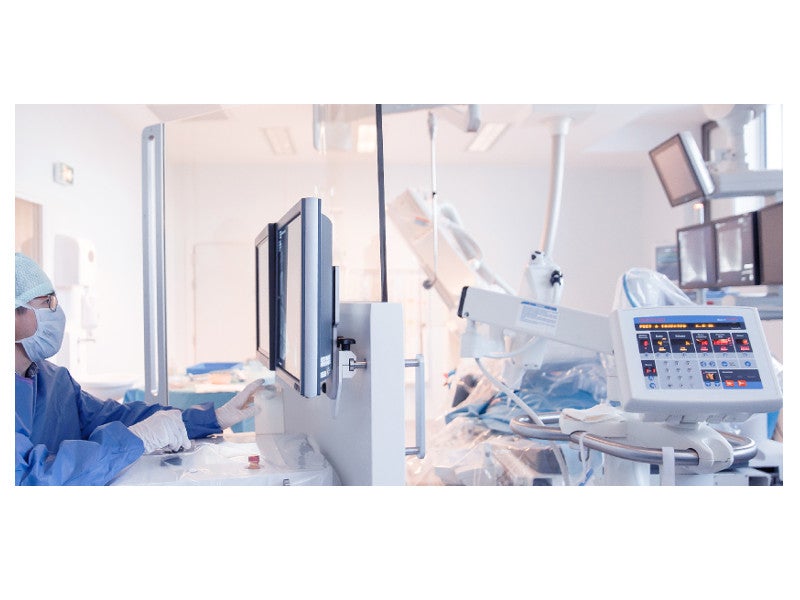
The R-One™ robotic device is a fully integrated robotics platform developed by Robocath for the remote delivery and adjustment of guidewires and stents during percutaneous coronary intervention (PCI).
The system received CE marking in Europe for the treatment of cardiovascular conditions in February 2019. It is one of the first robotic devices to have received CE marking in the interventional cardiology field.
Located in Rouen, the Medical Training and Testing Centre (MTC) will pioneer the use of the R-One robotic system for training future healthcare practitioners. Commercialisation of the device will be started in early 2019 in Europe and the Middle East.
Robocath’s R-One robotic device design and features
The R-One robotic device is designed to support the use of two stent or guide-balloon catheter units. It features a universal architecture, allowing compatibility with stents, guidewires and imaging systems.
The system can be installed and integrated quickly with the intervention protocol. It comprises a radio-protected control unit to protect healthcare practitioners from harmful X-rays and a telemanipulated robotic unit. These are coordinated by the command unit, which contains a catheter joystick, a guidewire joystick, a scopy pedal, a fluoroscopy command and C-arm command. It allows safe and accurate remote controlling of the instruments inside the vascular system.
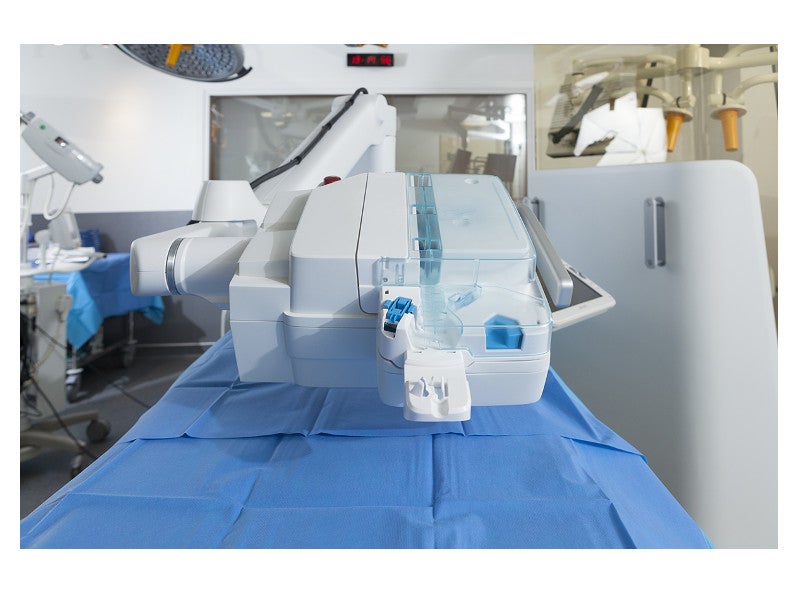
The telemanipulated robotic unit consists of a robot and the auxiliary support arm.
Details of the telemanipulated robotic unit in R-One
The telemanipulated robotic unit features a guidewire path, a guidewire clamp button, a catheter (balloon) path, release arm braking and a standby path. It includes various elements to streamline interventional vascular procedures using disposable Cassette sterile clipped system.
The guidewire path enables translational and rotational movements simultaneously. The stent or balloon path allows translational movements, while the standby path is used to put the guidewires and stent or balloon catheters on hold. The guidewire clamp button is used to securely lock the position of the guidewire in place during the procedure.
The release arm braking button helps control the movement of the support arm, enabling the physicians to precisely place the arm during the procedure.
The robot unit also features an emergency stop button to put the procedure on hold at any time.
Pre-clinical trials on R-One robotic device
The first pre-clinical study of the system was a prospective, randomised, controlled, multi-operator study that saw a total of 42 arteries stented. It was designed to evaluate the safety and efficacy of the system compared to the manual PCI in a porcine model.
The study has shown no significant difference between the procedures performed by the R-One robotic system and manual PCI.
R-One demonstrated 100% technical success with no major adverse cardiac events and no arterial damage.
Technologies utilised in R-One robotic device
The R-One robotic system is based on the R-grasp® and SecurAccess® technologies. R-grasp allows exact simulation of hand movements by the system, while SecurAccess ensures stability and security to the device during the procedure.
Other technologies include R-Lock®, which locks the guidewire in the robotic unit to significantly improve the robotic precision of the device. The possibilities for a new movement by the system such as continuous rotation are enhanced by Easy-Loop®.
The Radio-Stop® technology improves comfort and safety for clinicians when performing procedures, while East-Click® allows for the quick introduction of devices into the system and disposal before the procedure.