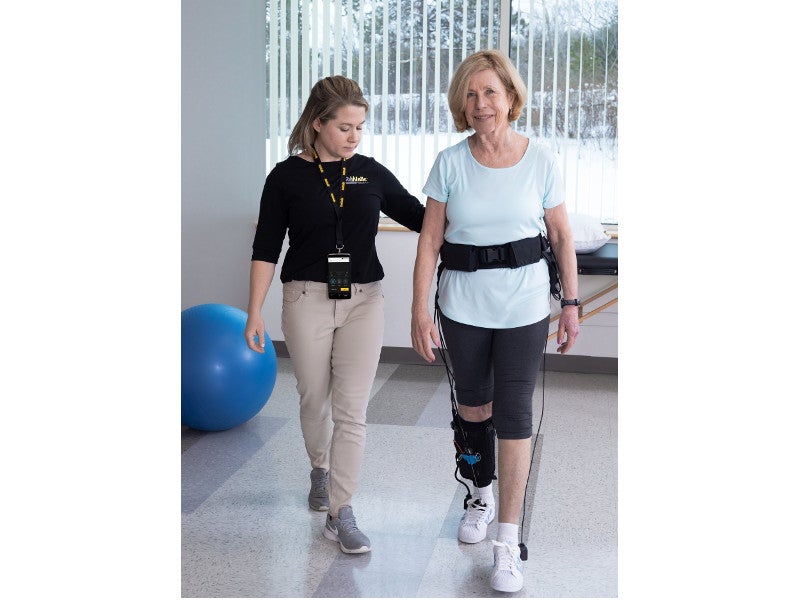
ReStore™ is the first wearable soft robotic exo-suit developed to treat mobility issues in post-stroke.
Developed by ReWalk Robotics, the soft exo-suit system is a clinic-based system designed to be used during gait rehabilitation therapy for individuals recovering from stroke.
ReWalk submitted 510k application to the US Food and Drug Application (FDA) in February 2019 for the ReStore. The device received clearance in June 2019 and received CE Mark in Europe in May 2019.
ReStore exo-suit design and features
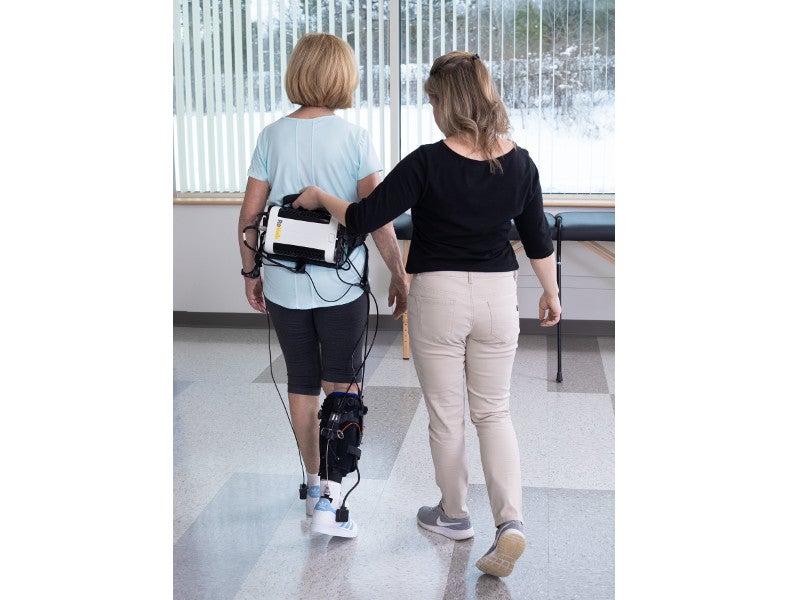
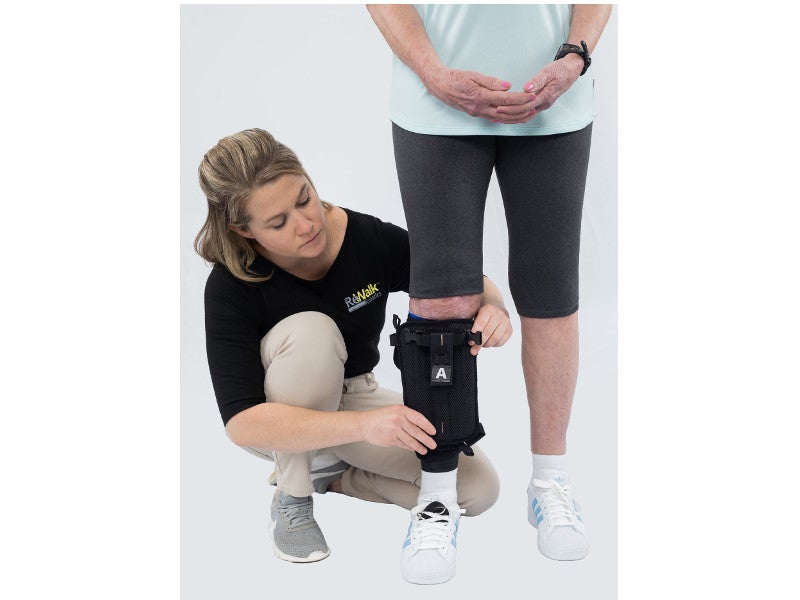
The ReStore™ system is a battery-powered, lightweight, soft, exo-suit. It comprises a fabric-based structure with a attached waist pack and mechanical cables. The waist pack is integrated with a control unit and can be adjusted by a waist belt, which also holds a support handle for therapists to provide additional guarding and support to patients.
The system provides plantar flexion (forward propulsion) and dorsiflexion (ground clearance) training, which integrates with the natural movement of the patient. The device is activated by the patient’s gait motion and adapts to synchronise with the gait.
The system can be used overground or on a treadmill, and accommodates walking speeds of up to 5km per hour. The ReStore device design allows sitting, standing, walking, and turning but does not support sports or stair climbing. It has a usable life of approximately three years, subject to timely servicing.
The system features versatility in rapid transitions between assisted and unassisted walking and accommodates supplementary support aids as required by the patient. It also provides measurable and quantifiable data and feedback in real-time to track a patient’s progress.
Additional features of ReStore exo-suit
The system communicates with a handheld device through Bluetooth Low Energy that uses 2.4GHz radio frequency for the control and configuration. It is powered by two rechargeable lithium-ion batteries.
The forces applied to promote ankle plantar and dorsiflexion are driven by motors and gears at the Waistpack and transmitted via Bowden cables to the textile components worn at the calf and foot..
ReStore exo-suit’s mechanism of action
The motors present in the waist pack of the device transfer power through the cables, while the sensors identify the gait events of each foot. The cable behind the calf contracts to pull the patient’s heel up and assists plantar flexion to propel the body forward, while the anterior cable contracts to lift the toes and provide assistance in dorsiflexion for ground clearance.
The non-paretic leg remains free for movement and, in case of imbalance, enables the patients to move and attempt a recovery on their own.
The system features two additional modes – Slack and Brace, which add to the range of capabilities for gait training. In case of any system error, it defaults to Brace mode and cables lock the ankle in a neutral position.
The ReStore™ mobile application provides real-time analysis and results of the training sessions.
Clinical studies on Restore exo-suit
The FDA clearance of the device was based on the results of a clinical study performed on 44 patients across five sites for four weeks. The complete study required seven visits to the centre.
The primary endpoint of the study was to evaluate the device’s safety during the gait training activities.
All the patients completed a few or all the walking activities in the system during the first visit. Out of 36 patients who completed all study activities, the majority increased their walking speed from first to the seventh visit.
In 10m walk tests with the device in Assist mode, comfortable walking speed was improved in approximately 86.1% of the patients, while 80.6% witnessed an increase in the maximal walking speed after the study.
Similar results were observed in patients’ walking speeds when measured during walking without the ReStore device, with 63.9% of patients experiencing an improved baseline comfortable walking speed and 77.7% of patients experiencing an improved baseline maximal walking speed following the series of training sessions with ReStore.
No device-related serious adverse events or falls were observed during the trial.
Marketing commentary on ReWalk Robotics
Founded in 2001, ReWalk Robotics is a medical device company with headquarters in the US, Germany and Israel.
The company is focussed on the development and manufacturing of wearable exoskeletons for people with lower limb disabilities. It markets the FDA-cleared ReWalk Personal 6.0, a exoskeleton for individuals with spinal cord injury, as well as the ReStore™ exo-suit.