The TiLink-P™ chronic sacroiliac (SI) joint fusion system is a first-of-its-kind standalone device designed to stabilise or fixate the ilium and the sacrum parts of the pelvic bone structure.
It was developed by SurGenTec, a medical device company.
The device is an advanced posterior SI fusion system that integrates effective compression, minimally invasive muscle-sparing posterior approach, and bone grafting to improve healing and patient outcomes in surgeries involving the SI joint.
The device received the US Food and Drug Administration (FDA) clearance in October 2023.
TiLink-P fusion system details
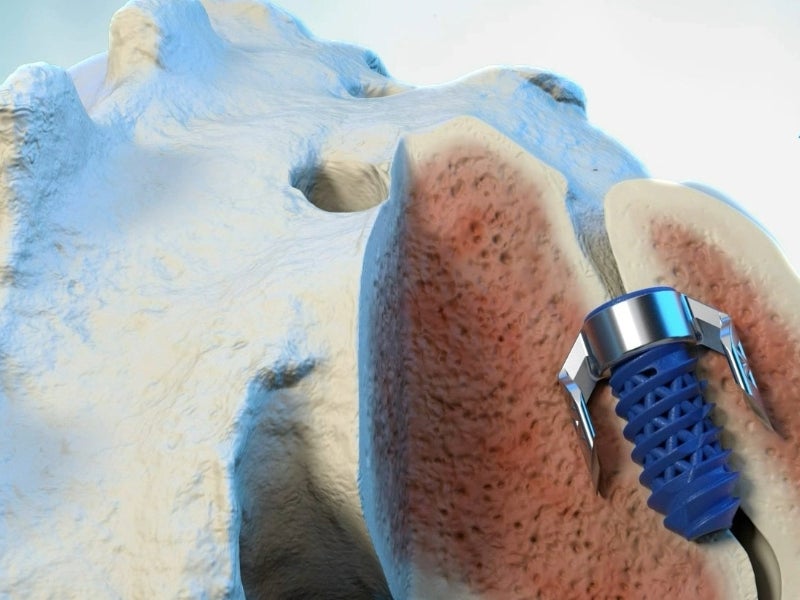
The TiLink-P novel fusion system comprises a transfixing compression anchor and a locking screw.
The system resists all SI joint forces, including shear, nutation, counternutation, compression and distraction. It offers osteocyte attachment and vascularisation.
TiLink-P is designed for minimally invasive surgery, requiring only one small incision.
The design reduces the procedure’s invasiveness, potentially leading to quicker patient recovery and less postoperative pain.
Compression anchor details
The anchor features bilateral cutting keels with bevelled tips and bone ingrowth windows.
The keels help to cut through bones. The curved structure of the anchor helps resist biomechanical forces.
The innovative double outer bevelled edging of the anchor helps generate substantial compression when inserted across the joint while the bone ingrowth windows allow for the dispensing of bone graft within and around the implant to enhance the fusion process.
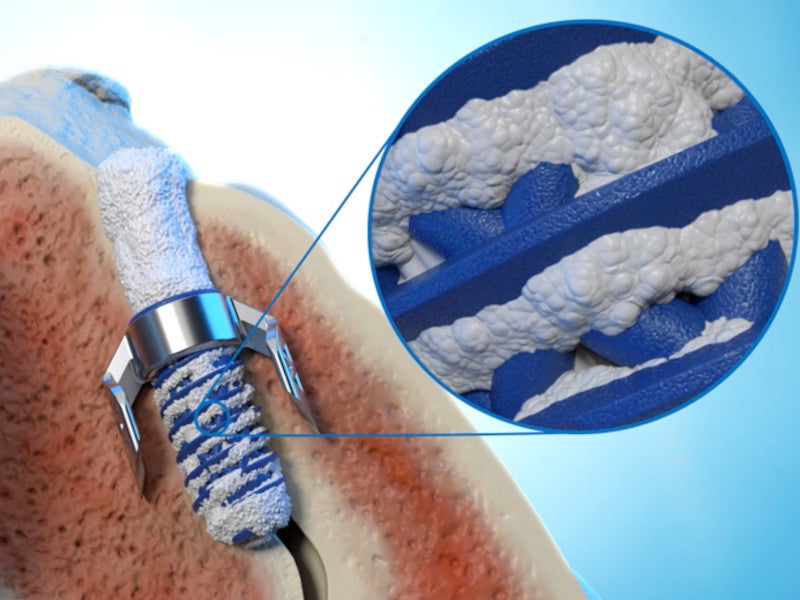
Locking screw details
The locking screw of the TiLink-P system is constructed from 3D-printed titanium. The material offers strength and durability while also incorporating Nanotex surface technology.
The hydrophilic surface created by Nanotex, a nanotechnology-based textile enhancements provider, enhances the implant’s osteointegration or the bonding of the implant with the surrounding bone.
The open trellis design features 500μm to 750μm openings to facilitate bone ingrowth throughout the screw, enhancing the potential for fusion.
It offers a maximum biologic potential of up to 5cm3 of biologic post-packing.
Self-harvesting channel, self-tapping tip and a cannulated screw are some other features of the locking screw. The design prevents implant migration.
The harvesting channel collects autograft as the screw is inserted, aiding in fusion. The screw is cannulated to allow the packing of bone graft.
Simulated and cadaveric biomechanical evaluation on TiLink-P fusion system
Rigorous biomechanical testing and cadaveric studies have validated the TiLink-P fusion system’s effectiveness and safety, providing confidence in its clinical application.
The innovative device has the potential to simplify surgical procedures, enhance patient recovery, and reduce hospital stays.
The biomechanical evaluation was conducted to assess the compressive properties of the TiLink-P system under extreme physiological loads and its ability to maintain compression across the SI joint when implanted using a posterior in-line surgical approach.
In the initial simulated joint assessment, the TiLink-P was implanted along the longitudinal axis of a simulated sacroiliac joint.
Compressive forces at the implant and bone foam interface were measured before and after implantation under static physiological loading. Results showed a significant increase in compressive forces, exceeding a 500% rise post-implantation.
The TiLink-P exhibited compressive forces across the simulated SI joint that were five times greater than the pre-implantation state just one hour after implantation.
Subsequently, a cadaveric biomechanical assessment was performed on eight human cadaveric lumbosacral spines with attached pelvises.
The study involved non-destructive cyclical axial loading to evaluate long-term changes in gap spacing across the SI joint.
Each specimen received one TiLink-P implant on one side and a previously FDA-cleared predicate SIJ fixation screw on the opposite side. Micro-transducers measured the gap spacing before and after both the implantation and cyclic testing.
The findings revealed that the TiLink-P system effectively compressed the SI joint upon implantation and sustained this compression throughout the duration of cyclic loading, even under supraphysiological loads.
The bevelled design of the TiLink-P anchors was noted to contribute to the maintenance of compressive properties across the joint.
Marketing commentary on SurGenTec
Headquartered in Boca Raton, Florida, US, SurGenTec is actively engaged in developing and manufacturing a wide range of innovative products, including implants, instruments, biologics, and ancillary solutions.
SurGenTec is collaborating with navigation companies to integrate TiLink-P with platforms that provide pinpoint accuracy during implant placement.
The collaboration will ensure optimal safety and stabilisation for patients.
In March 2024, the FDA cleared the company’s OsteoFlo HydroPutty Synthetic Bone Graft, which marks a significant advancement in the company’s bone graft technology.