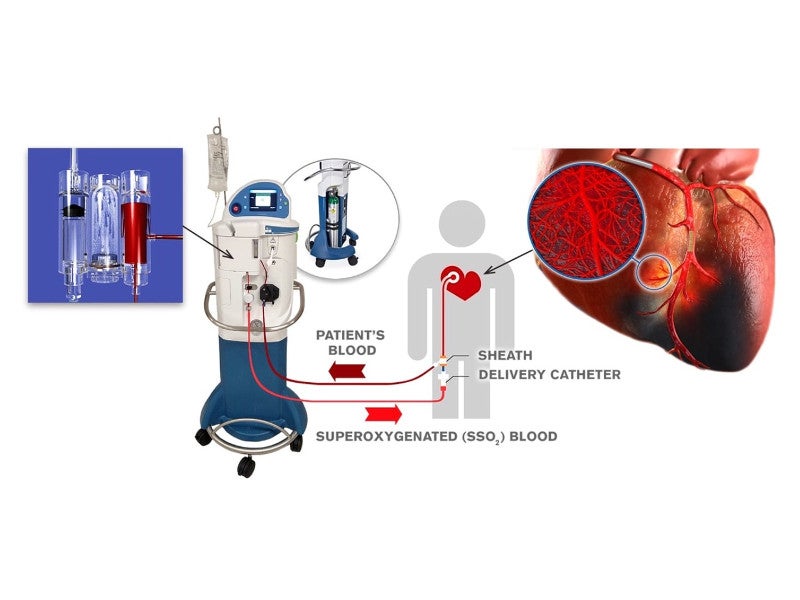
TherOx’s SuperSaturated Oxygen (SSO2) Therapy is delivered by its DownStream® system, which creates and delivers the patient’s super-oxygenated blood to the targeted ischemic regions of the heart in patients with acute myocardial infarction (AMI).
The device received pre-market approval from the US Food and Drug Administration (FDA) for clinical use in April 2019. It is claimed to be the first and only FDA-approved treatment to reduce muscle damage in heart attack patients beyond percutaneous coronary intervention (PCI), which is the standard-of-care treatment.
TherOx DownStream system design and mechanism of operation
TherOx DownStream system comprises the main unit, a cartridge, and an SSO2 delivery catheter, which work together to increase blood circulation.
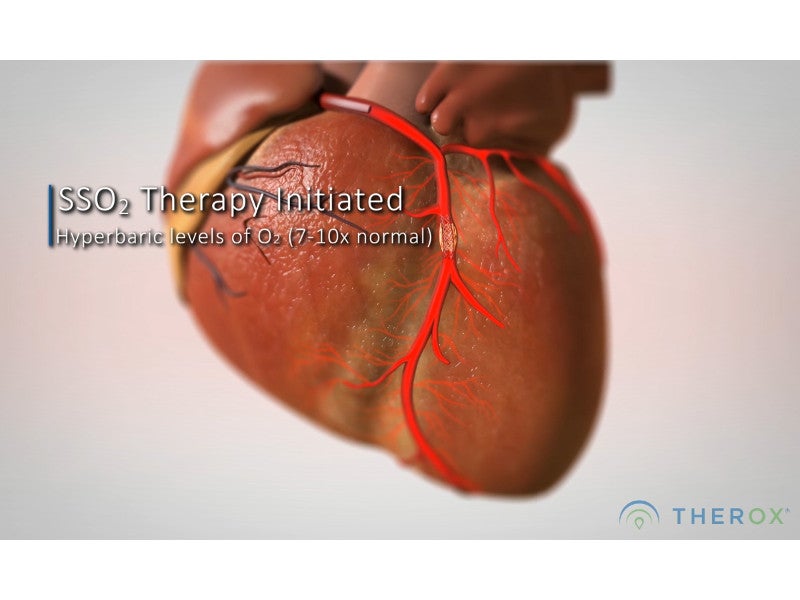
The device is based on the hyperbaric oxygen treatment concept, which aims to support the body’s natural healing abilities. It mixes highly oxygenated saline with the patient’s blood and delivers it directly to targeted ischemic areas of the heart through catheters, within six hours of developing symptoms.
The one-time, 60-minute process restores normal oxygen levels in the heart and improves blood flow in the surrounding areas. The infusion is delivered immediately after the PCI to improve microvascular blood flow in patients, reducing the infarct size and saving heart tissue.
DownStream system unit features
The DownStream cartridge unit is a sterile, single-use, disposable system made up of injection-molded polycarbonate. The unit comprises the blood-contact fluid flow path and the SSO2 delivery catheter.
The tubing material of the unit is approximately 8ft-long and made of polyvinyl chloride. The blood priming volume of the unit is approximately 60ml.
The unit prepares the SSO2 solution from the oxygen gas supplied by the hospital and sterile saline solution. It also delivers the patient’s arterial blood to be mixed with the SSO2 solution to form the oxygen-rich hyperoxemic blood.
DownStream cartridge unit features
The DownStream cartridge unit is a sterile, single-use, disposable system made up of injection-moulded polycarbonate. It is inserted into the DownStream system for SSO2 therapy. The unit comprises the blood-contact fluid flow path and the SSO2 delivery catheter.
The tubing material of the unit is approximately 8ft-long and made of polyvinyl chloride. The blood priming volume of the unit is approximately 60ml.
The unit prepares the SSO2 solution from the oxygen gas supplied by the hospital and sterile saline solution. It also delivers the patient’s arterial blood to be mixed with the SSO2 solution to form the oxygen-rich hyperoxemic blood.
Details of the SSO2 delivery catheter unit
The delivery catheter is a 100 cm-long over-the-wire unit that features an end-hole for the output of fluid, along with a standard luer fitting at one end to connect it with the return line of the cartridge.
The catheter is introduced in the ostium of the left main coronary artery of the patient and produces circuit pressure between 1,000mmHg and 1,400mmHg, with return blood flow rate of 100ml a minute.
Clinical studies on TherOx DownStream system
The FDA’s approval for the system was based on the results of multiple clinical trials, including the pivotal AMIHOT II trial, which enrolled 301 patients with anterior acute myocardial infarction and the IC-HOT study, which enrolled 100 patients.
In AMIHOT II, the patients were randomised to receive either SSO2 therapy immediately post PCI or PCI alone. The primary effectiveness endpoint of the study was infarct size reduction. The trial demonstrated a 26% reduction in the patient’s infarct size compared to those receiving PCI alone.
The IC-HOT study confirmed the safety and effectiveness of SS02 Therapy in treatment of LAD STEMI patients who underwent successful PCI with stenting within six hours of experiencing symptoms.