
Oncology is the most active area in clinical trials globally this year, as more resources than ever are being dedicated to developing and testing new cancer treatments.
At the same time, there is the recurring issue of patient recruitment and retention, which has become heightened due to the complexities involved with oncology clinical trials.
All the more reason why, coming out of the global pandemic, contract research organizations (CROs) must optimize resources, manage supply chain disruption, and leverage technology solutions to help simplify that complexity and optimize workflows.
“Oncology trials are inherently complex because it’s a progressive disease and the way you treat it varies dramatically depending on how a particular participant may respond,” explains Walker Bradham, Zelta product leader at Merative. “So, designing the trials to mimic that real-world reaction to a therapy becomes challenging.”
And as personalized medicine has become more mainstream, cancer research is benefiting with increasing understanding of oncogenic drivers resulting in more targeted therapies. This increase in more targeted therapies fuels additional complexity in trial designs, and increases the challenges in trial execution.
What the data shows: Oncology clinical trials outpace all other therapeutic areas worldwide
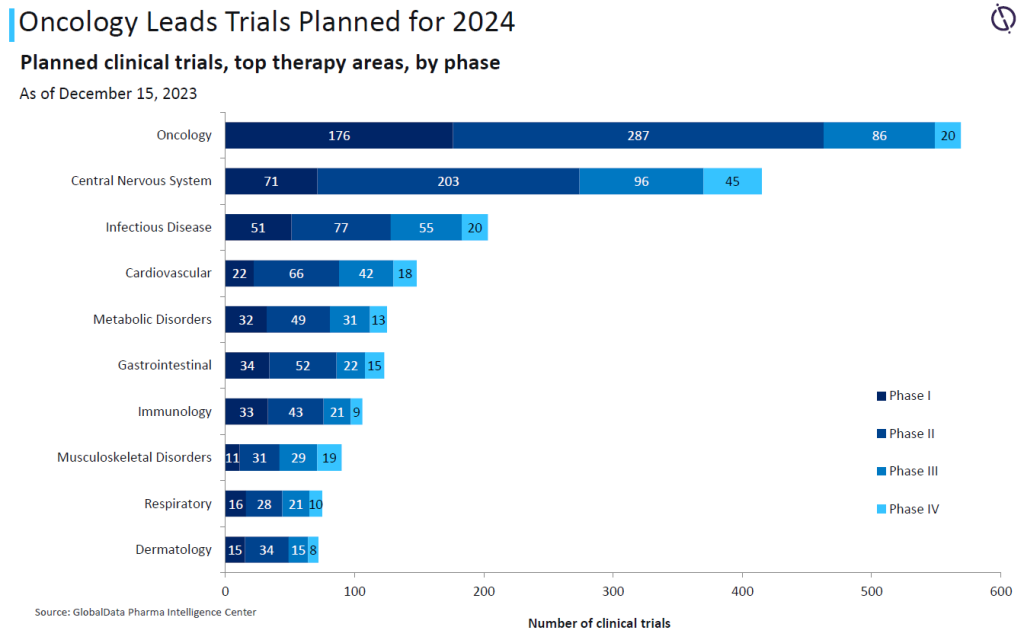
CROs are going to be in demand for oncology trials this year. Data from GlobalData’s Clinical Trials Intelligence Center highlights how oncology is the top therapy area for both trials planned or expected to complete in 2024.
Of the 6,528 oncology trials expected to wrap up this year, 57% are in Phase II, more than double the 25% of trials in Phase I. The total of oncology trials far exceeds the next therapy area of central nervous system treatments, which has 4,043 trials expected to complete this year. These figures roughly continue the trend from clinical trials projected to complete in 2023.
For studies starting over the next year, oncology continues to be the dominant therapy area with 569 trials, well ahead of central nervous systems products at 415 trials, and infectious diseases with 203 trials.
While pain treatments top the indication list for planned trials this year with 105, oncology studies occupy six of the remaining top 10. Solid tumor treatments have the second-most planned trials in 2024 at 82, with 41 in Phase I and 37 in Phase II.
Non-small cell lung cancer has the third-most planned trials at 65 with almost 54% in Phase II. Meanwhile, breast cancer trials have the fourth highest total with 57 set to begin in 2024.
The three remaining cancer types with trials in the top 10 are colorectal cancer, acute myelocytic leukemia, and pancreatic cancer. Notably, acute myelocytic leukemia and pancreatic cancer were not on the top 10 indications list for trials planned last year. Their inclusion gives hope to patients living with these difficult-to-treat conditions.
In recent years, decentralized clinical trial (DCTs) concepts such as eConsent, ePRO, eCOA, wearables, in-home monitoring devices and televisits have become more widely adopted. The aim being to reduce study participant burden, increase engagement, and offer access to new endpoints.
Yet of the total trials expected to complete this year, just 7% have a DCT component, and within these trials, only 7.2% are for oncology. Given the continued and increasing investment in oncology research, along with the increase in more targeted research studies, DCT solutions can help reach and retain key patient populations.
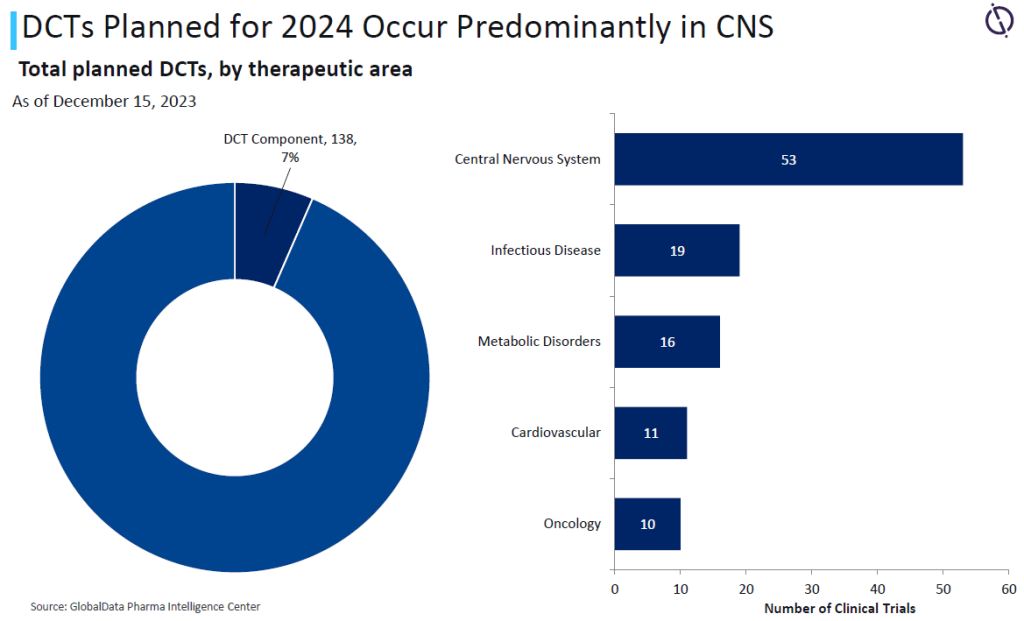
Due to the method of treatment, full DCTs are impractical for oncology. However, there is still potential for using hybrid techniques in engaging study participants, provided sufficient technological support is available.
In line with the growth of oncology trials, CROs are developing ever more innovative ways to facilitate patient participation and data collection for a smooth trial process, as well as to optimize resources and budgets. This includes using more flexible and adaptable technology solutions and working with more of an intelligent ecosystem mindset.
How CROs can use tech to optimize workflows in oncology
The World Health Organization has recently predicted a 75% rise in global cases of cancer by 2050. Given the growing prevalence of cancer among aging populations, CROs are more important than ever to advance clinical trials – playing a key role in the strategic planning of trials, patient recruitment, data analysis, and quality assurance.
However, as experienced throughout clinical research, CROs also face challenges with hiring and retaining trained talent in a post-COVID world. As a result, CROs have become increasingly reliant on leveraging tech capabilities that are more intuitive, i.e. easier to train, and provide optimized workflows that drive efficiency and allow CROs to do more with less.
One such tool for doing this is Zelta, a clinical data management and acquisition platform from Merative. The cloud-based platform has been designed using software-as-a-service (SaaS) principles to ensure that CROs have control of their clinical trials at all times and have confidence in the accuracy and integrity of outcomes.
To that end, Zelta helps data management teams configure dynamic event schedules, navigate mid-study updates (such as protocol amendments or design usability improvements), automate repetitive tasks, deliver real-time insights to inform workflow, and generate datasets ready for analysis.
These capabilities enable CROs to more efficiently coordinate data collection, adapt as needed, and reduce the long cycle-times typically associated with traditional development methodologies for Oncology trials.
One way that systems like Zelta have made a material difference is by offering modern responsive user experiences. These techniques typically involve persona-based screen and workflow designs along with real-time edit checks of user entered data (also known as client-side validation).
“CRO and sponsor partners point to site burden as one of the main obstacles to trial success. Creating more familiar, modern designs can reduce cognitive load and drive real results. Our analysis of legacy offerings revealed that 30% or more of edit check queries in an EDC are closed within an hour of creation,” explains Bradham.
“Though that might sound like a positive metric, it actually causes quite a bit of burden on the sites and data management teams. Addition clicks, a muddy audit trail, and the potential for reason for change documentation. Modern techniques of real-time data validation during data collection informs the user of potential mistakes on the spot, drive accuracy, and reduces cycles.”
The modernized user experiences in Zelta also ease navigation and can instantly flag potential inaccuracies during data collection, reducing the burden on researchers. These methods, offered out of the box with Zelta, have proven to reduce study timelines and improve data quality.
Simplifying clinical trials for oncology
The Zelta platform helps accelerate study start timelines and reduce mid-study update downtime for oncology trials, and simplify the trial execution. For example, the platform can accommodate a cycle-based oncology clinical trial, which could include three treatment cycles that are repeatable or four events per cycle on specific days of the trial.
Comparable systems which lack native cycle capabilities require either exorbitant amounts of configuration (hundreds of study event and rule combinations) or custom programming. Both lead to cost to implement, additional effort from study teams to validation, and exposure for unexpected delays and expenses if mid-study changes are needed.
“We’ve been able to learn from how trials have been designed in the past and have structured our underlying data model to support these complex use cases, allowing the study designers to build in the predictable amount of cycles, include dynamic logic for the system to trigger additional cycles, plus the flexibility for the investigator to add cycles on-demand depending on the protocol. All while the data flows in real-time to a harmonized database that CROs can analyze in a timely manner,” adds Bradham.
“On top of that, we can design these studies so that they can be adaptable. As seen in some platform trial or advance protocols, you could branch certain study participants into an entirely different study design that may align more closely with how they’re being treated.”
CROs are facing a banner year for oncology clinical trials – a greater demand for trials, outpacing all other therapy areas, requires more resources than ever before amid post-pandemic supply chain disruptions. Zelta empowers CROs to overcome these challenges, with capabilities for adapting to changing circumstances and meeting the specific needs of patients throughout an oncology clinical trial.
Learn more about how CROs can leverage Zelta for their 2024 clinical trials.



