Medical Device Development and Clinical Evaluation - Clinical Trials
Goal: Give some high level insight in the obtaining of clinical data following the MDR-regulations. To read more please download this free white paper.

The name BAAT is a Dutch word that translates into ”Benefit”. There are three benefits (advantages) that BAAT Medical offers to customers around the world: Usefulness, Physical improvement and Profit. your medical device
You have successfully submitted your enquiry. Someone from our company will respond ASAP
The name BAAT is a Dutch word that translates into ”Benefit”. There are three benefits (advantages) that BAAT Medical offers to customers around the world: Usefulness, Physical improvement and Profit. A range of medical device development companies rely on the services that BAAT offer, such as innovation and engineering services to medical device manufacturing. The full scope of medical device engineering services include product realization, maintaining, clinical evaluation, clinical trials, PMCF and all regulatory related services to maintain compliance for the FDA and notified bodies.
Starting a Medical device development project is like assembling a complex puzzle, involving many stakeholders. BAAT provides a range of services and expertise (puzzle pieces) that help medical innovations come to market and projects move forward. BAAT interconnects these service areas to work towards a successful product launch. The specific areas of expertise and experience can be divided into six services:
What differentiates BAAT from regular medical consultancy companies specializing in certain topics, is that BAAT covers the whole scope. This enables full lifecycle medical product development projects and supply chain management solutions. BAAT interconnects the important aspects and defines the best possible innovation-route to attain a commercially viable medical product.
BAAT assures meeting all legal requirements for product safety and regulatory approval and can bear legal responsibility, handle warranties and liabilities correctly within the medical supply chain.
Customers obtain total medical compliance and BAAT has the operational and hands-on experience to develop, manufacture and maintain a medical device in the field.
From the onset, most of our customers bring in at least a concept start with. This can be a fully developed product or just a sketch, and everything in between. However, often new concepts are developed with today’s world in mind but to have impact, products should be meaningful for tomorrow’s patients in tomorrow’s world to make it an innovation instead of an improvement. We help you to get clarity on this and create the best starting point for realizing an innovative, meaningful, and futureproof product that also makes sense from a commercial perspective.
At BAAT we have all capabilities in house to develop your medical device from initial idea to a finished product and we can organize and validate all processes for manufacturing. We make sure that apart from delivering the product, we also deliver all documentation needed, ready for submission at the competent authority. We adhere to our development process while maintaining the flexibility to tailor the specific needs of our customers.
Once the device is approved for market and has a go for launch, manufacturing can be initiated. For a medical device it is extremely important that the device is manufactured according to the applicable regulations. BAAT manages your complete manufacturing chain and makes sure that all associated records are complete and valid. We evaluate and handle non-conformities in deliveries and deviation requests coming from suppliers.
We monitor all the special processes within the manufacturing chain like cleaning and sterilization and take care of supplier audits that are required.
At BAAT we understand that changes in products and processes are inevitable, especially when a product is brand new. Changes should be implemented correctly while always taking patient safety into account. We structured our processes in such a way that such changes are implemented and recorded properly according to the regulations.
According to the medical device regulations a medical device should be developed and manufactured following a controlled process. In our opinion, this does not mean that you should always implement and maintain an expensive Quality Management System yourself. Together with our customers we find efficient ways to fulfil the quality and regulatory requirements. One of the possibilities we offer is to connect your Quality system to ours and take advantage of our already implemented procedures for development and manufacturing. This approach will reduce your initial investment and also your maintenance costs while still being able to act as legal manufacturer for your product. For startups, it means they can start implementing a Quality Management System only when it becomes relevant.
Patient safety in our approach, is part of the development process right from the start. It is a central element in medical device development where risk management, end user information, clinical evaluation and post market surveillance activities are interconnected. We develop the clinical evaluation plan and deliver all results you need for market approval and product maintenance.
When a premarket clinical investigation is required, we work together with the appointed CRO. Together with you we ensure a right balance between the clinical benefit that you want to claim and the effort it takes to provide the clinical evidence for those claims.
We help our customers to understand and define the important topics that should be covered in contracts throughout the manufacturing chain. This part of the development process of a medical device is often overlooked and underestimated. Our approach is very practical and concentrates mainly on three topics: Liability, Warranty and Insurance. Together with our customer we evaluate potential liability and commercial risks and implement measures and processes to mitigate those risks.
If needed we are even able to take over the formal responsibility of acting as legal manufacturer on behalf of our customer.
BAAT has a multidisciplinary team with 45+ employees available for you, including mechanical, biomedical, mechatronic and quality engineers supported by subject matter experts regarding clinical, risk management, regulatory, useability, biocompatibility, verification testing, project management, statistics, prototyping and industrial design.
Since our start in 1999 we have helped both small and large customers worldwide to generate innovative medical devices, from class I to class III. Our head office is in Hengelo, the Netherlands and we have a subsidiary in Minneapolis, USA.
We offer you a complete range of services to identify, realize and maintain your medical device.
Contact us to see how we can help you best.

Goal: Give some high level insight in the obtaining of clinical data following the MDR-regulations. To read more please download this free white paper.

BAAT has successfully passed the ISO 13485 recertification audit by our Notified Body MedCert with ZERO non-conformities! This achievement re-secures our ISO 13485 certification, valid until November 24, 2027.
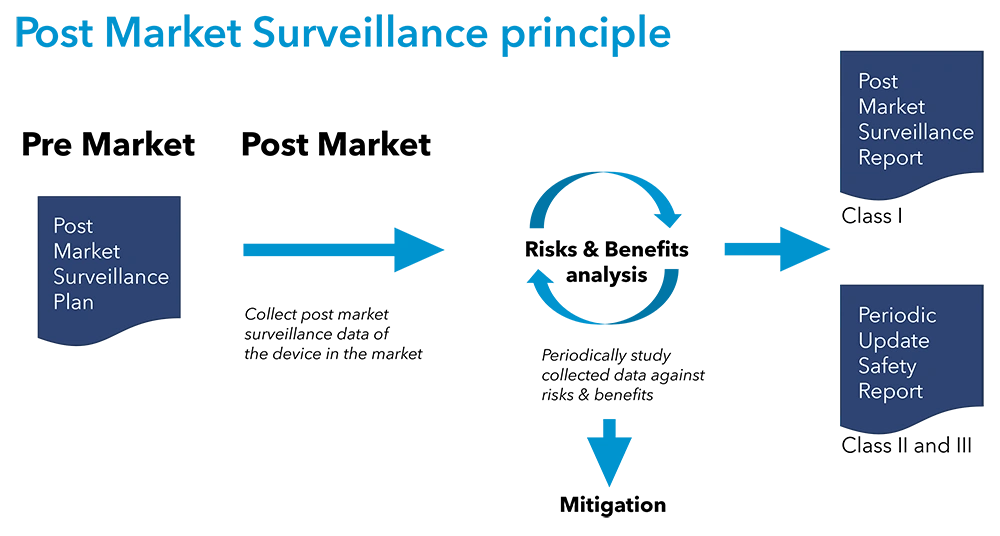
The landscape of medical device regulation in the European Union (EU) has undergone significant changes with the implementation of the Medical Device Regulation (MDR) and In Vitro Diagnostic Regulation (IVDR).

BAAT Medical Products and Witec Medical are developing an innovative approach aimed at accelerating and making the validation and production processes for medical products more cost-effective.

Assuming that simply following a development process stage by stage will lead to success in MedTech product development is untrue.
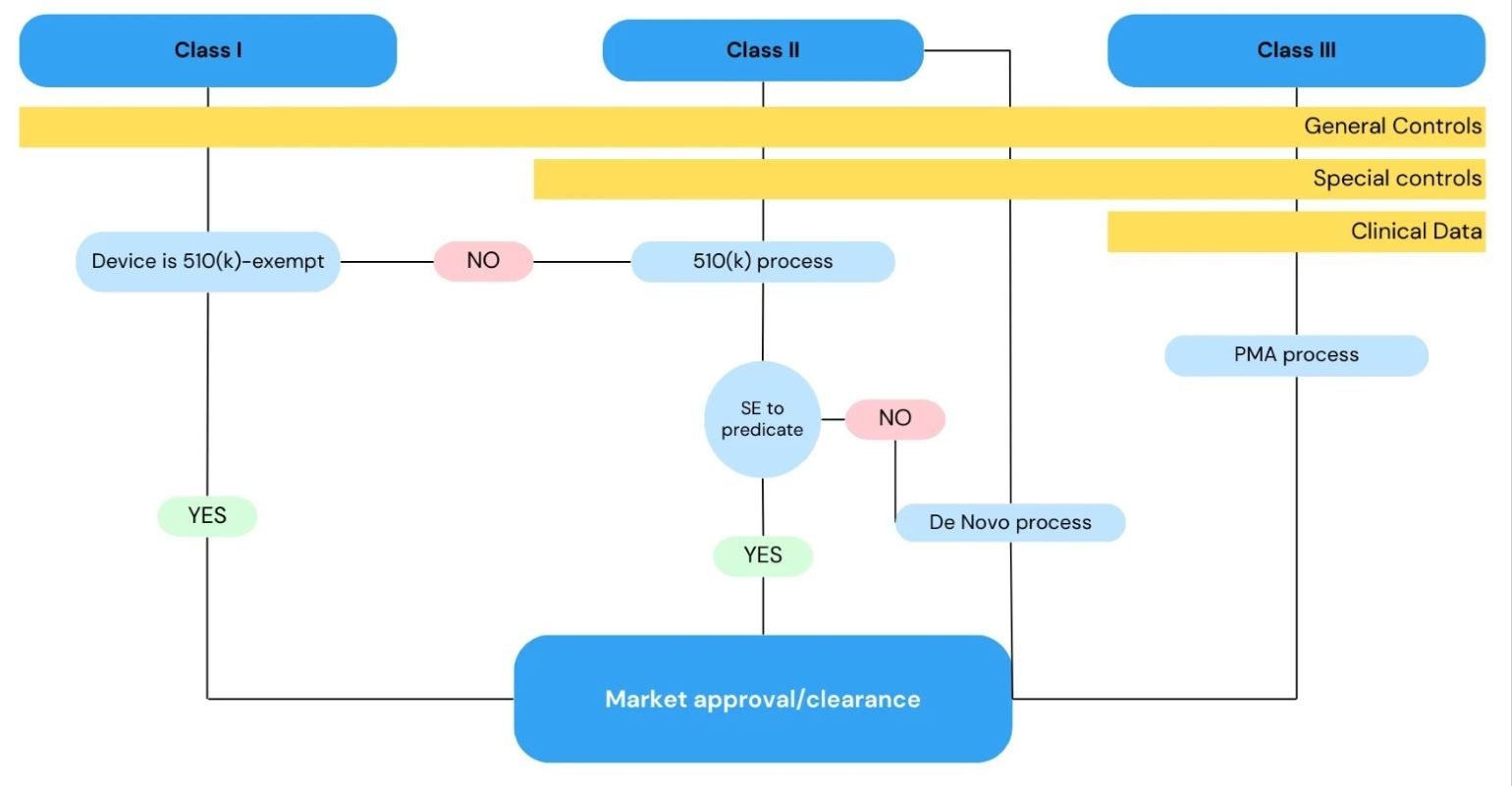
The FDA employs a risk-based approach to regulate the introduction of medical devices, including orthopaedic implants, into the US market.

“My device consist only of titanium, stainless steel or PEEK, and therefore it is biocompatible”, we have heard it once and again, but it cannot be further off from the truth.