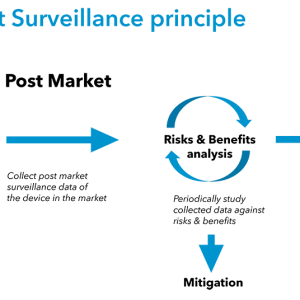
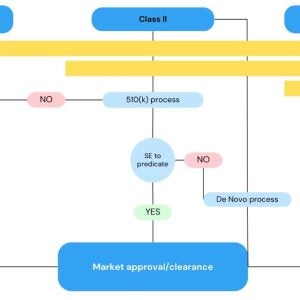
BAAT Medical passed the ISO 13485 recertification audit!

BAAT has successfully passed the ISO 13485 recertification audit by our Notified Body MedCert with ZERO non-conformities! This achievement re-secures our ISO 13485 certification, valid until November 24, 2027.
Why does this matter for you?
Navigating MDR compliance (EU Medical Device Regulations) is one of the toughest challenges for Medtech startups. When partnering with BAAT’s MDR-compliant processes, you gain:
- A faster path to market, reducing costly delays
- Confidence in a robust ISO 13485 Quality Management System
- Support for seamless regulatory compliance in the Medtech sector
BAAT’s solutions are tailored for Medtech innovators. We help you focus on scaling your business while we handle the complexities of compliance.
Ready to accelerate your Medtech journey? Contact us at info@baatmedical.com!