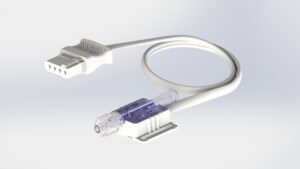
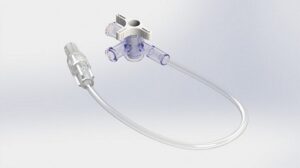
Product Customisation
Customising products to fit different needs of various applications and usage is a basic requirement of most manufacturing companies and in particular medical device manufacturers.
Medical device companies are consistently searching to develop new solutions for improved procedures and more effective patient treatment. The innovation of new solutions often requires customised devices and components, which in many cases are small but essential parts of the whole new product development.
Some manufacturers use in-house customisation capabilities while others outsource projects to companies that have expertise in specific product lines, especially when it comes to smaller components and devices. To make it feasible for a company to invest such efforts in a specific component or a production line, it must be a company that focuses on a relatively niche product line.
When it comes to medical product customisation, being able to customise products is good, but to truly be a significant partner in developing new systems, one needs to be familiar with the clinical area and have vast experience and internal knowledge in product customisations.
Furthermore, product customisation processes require significant documentation in order to receive regulatory approval. In the medical device field, this requirement is trifold – as each change requires regulatory approval and documentation according to ISO standards, biocompatibility, shipping tests and more.
Elcam Medical is a world-class producer of disposable medical devices and accessories for the OEM market, and a provider of innovative solutions for specialised flow control needs. Being an OEM company, the capability to customise our products to fit new developments is one of our most important assets.
Elcam’s focus on disposable components for original equipment manufacturers (OEMs) enables us to invest a lot of effort in this line, which led us to have the broadest portfolio of stopcocks, manifolds and connectors for IV and drug delivery, with more than 1,000 stock-keeping units (SKUs) for the stopcocks line alone.
Elcam does not work as a subcontractor. We treat our customers as partners and share our extensive knowledge in product development and our long-standing clinical know-how gained in more than 40 years of experience. The Elcam teams know how to recognise market needs and recommend solutions, making us a significant partner in the development process.
Furthermore, as a true OEM company, Elcam’s quality assurance and regulatory affairs department provides customers with comprehensive regulatory support, including all the required documentation.