Mi3 is a specialist partner in the design, development and manufacture of complex plastic medical, pharmaceutical and scientific devices.
The company supports clients from an early stage concept interpretation to development and design for manufacture, ensuring high quality and cost-effectiveness through meaningful engagement. It also aims to prepare and develop the required technical documentation with ever-evolving regulations.
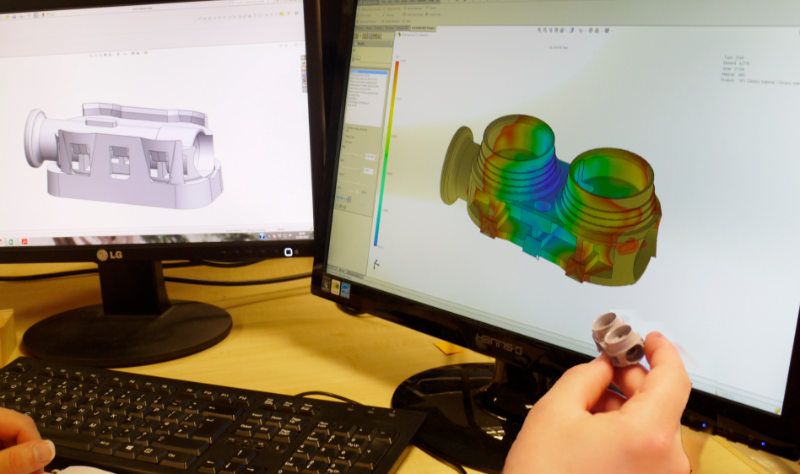
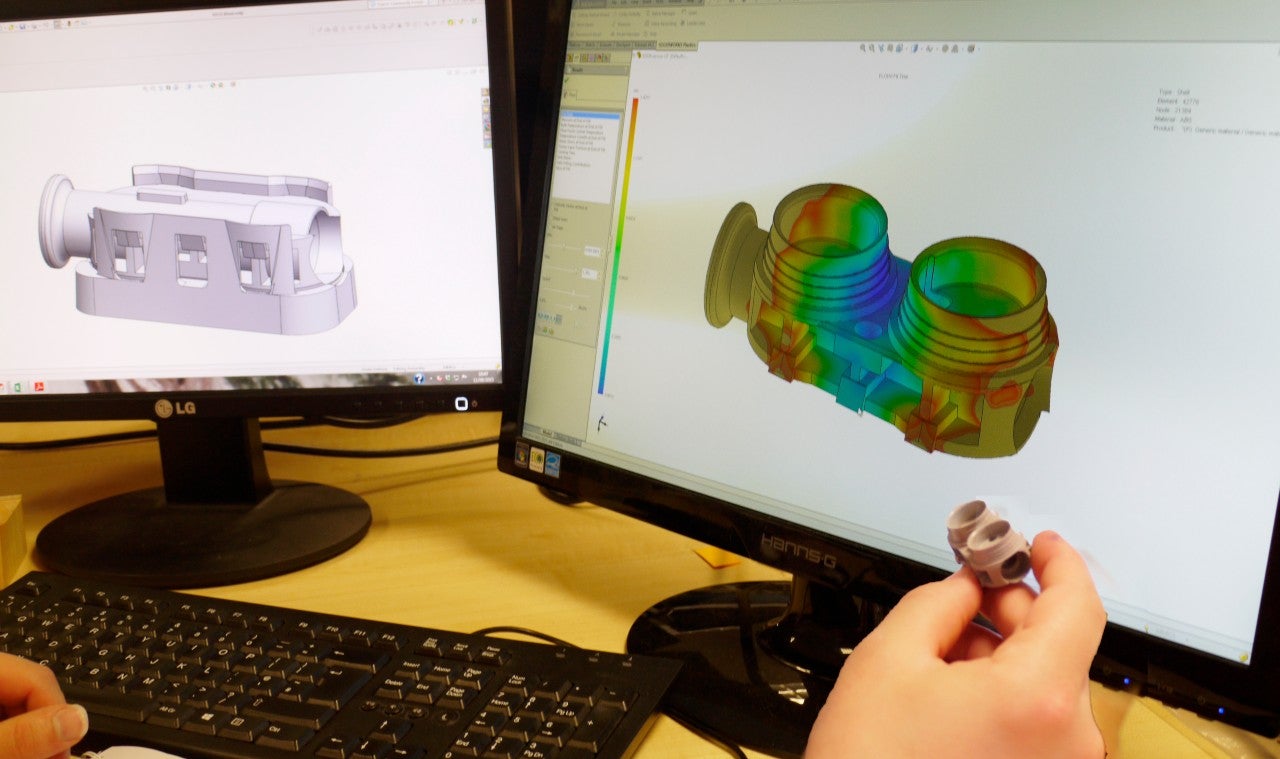
Innovative complex plastic medical device product development
Mi3 approaches each product design creatively, researching and using advanced materials, as well as the latest 3D design software, and priding itself on its reputation to offer a professional and enjoyable experience to its clients.
The company offers advice and inspiration when developing the initial product concept and design, continuing its involvement through to product realisation and marketing.
Complex plastic medical devices manufacturing
Mi3 allows flexibility and agility while still managing to produce more than two million items annually. With a passion for perfection, the company commits to providing best practice throughout its services.
The company’s extensive knowledge offers technical know-how and valuable insights. Its assembly cleanroom is classified level 7, and its processes and systems are accredited to ISO 13485:2016 and MDSAP. Injection moulding and extrusion is performed in a class 8 cleanroom.
Continuous improvement is a pivotal aspect of company culture; Mi3 encourages and trains its employees to explore better ways of practice daily, investing in training and processes to increase quality and efficiency, and driven to become a premier supplier of outsourced manufacturing in the medical devices sector.
Welfare of healthcare professionals and patients
Client satisfaction is integral to Mi3, which cares about the welfare of patients and healthcare professionals using its products, and taking pride that those products can significantly impact the lives of others.
Mi3 aspires to world-class standards with its clients, remaining responsive and adaptable while offering the highest quality with ‘On-Time in Full’ delivery, supported by performance measures owned and monitored by the company.
Complex plastic medical devices made with integrity
The company offers complete confidentiality and discretion to its clients, guaranteeing privacy and security with commercial and technical information, and responding promptly on commitments and decisions.
Mi3 guarantees its employees deliver its key performance indicators (KPIs) regarding quality, health and safety, and on-time delivery, its policy to deal rapidly with all client issues.
The company also actively explores changes in legislation while offering up-to-date, robust solutions to end-users.
Mi3 has a culture of total responsibility, remaining accountable to not only its clients but also internally, empowering members of its team to guarantee collective responsibility.
Collaboration with commercial partners
The company works in a trusted and shared partnership alongside its commercial partners and employees, working in an honest, open and respectful manner.
Furthermore, it has a close alliance with its clients, discussing specific requirements and customising its service accordingly, keeping in regular contact throughout the entire process, and informing product progress and delivery schedules.
Compliance with medical device regulations and standards
Mi3 are compliant with the International Organization of Standardization (ISO) standards and regulations required for each unique device and the countries of sale.
The standards and regulations the company regularly adheres to are:
- 2017/754/EU Medical Device Regulation (MDR) (EU)
- ISO 10993 Biological evaluation of medical devices
- ISO 11135 Sterilisation of healthcare products – Ethylene oxide
- ISO 11137 Sterilisation of healthcare products – Radiation
- ISO 11607 Packaging for terminally sterilised medical devices
- ISO 11737 Sterilisation of medical devices – Microbiological methods
- ISO 13485 Medical Devices – Quality Management Systems
- ISO 14644 Cleanrooms and associated controlled environments
- ISO 14971 Medical devices – Application of risk management to medical devices
- MDSAP Medical Device Single Audit Program
Medical device development from prototype to regulatory approval and distribution
Mi3 can be contracted for one or more parts for the product cycle or the entire process. The company can effectively take a new concept right through the product creation cycle from technology research, product design to finish with a fully validated device ready for distribution to the market.
Mi3 can assist with:
- Market and technology research
- Concept creation and design
- Development and prototyping
- Pilot to full-scale production
- Sterilisation
- Regulatory and technical documentation
- Warehouse and medical device distribution
Founded in 2006, Mi3 is recognised for its quality, integrity, innovation and customer care.
Growing successfully, the company has an international client base, continuing to operate on the same values that helped to build its reputation for excellence: innovation, excellence, customer focus, integrity and collaboration.