Oncgnostics designs in-vitro diagnostic tests for the detection of DNA methylation, which is associated with a wide variety of cancers.
The company uses these reliable epigenetic biomarkers to create products such as GynTect®, which is a clarification test for cervical cancer and its precursors. Results are rapid, allowing clinicians to detect cancers in early stages of development.
Oncgnostics implements an ISO: 13485 certified quality management system in its research, development and manufacturing processes.
Fast and reliable clarification in cervical cancer screening
GynTect is a fast and non-invasive test for cervical cancer suggested for patients with an abnormal Papanicolaou (Pap) smear or positive human papillomavirus (HPV) test results where further diagnostics are required such as colposcopy and biopsy.
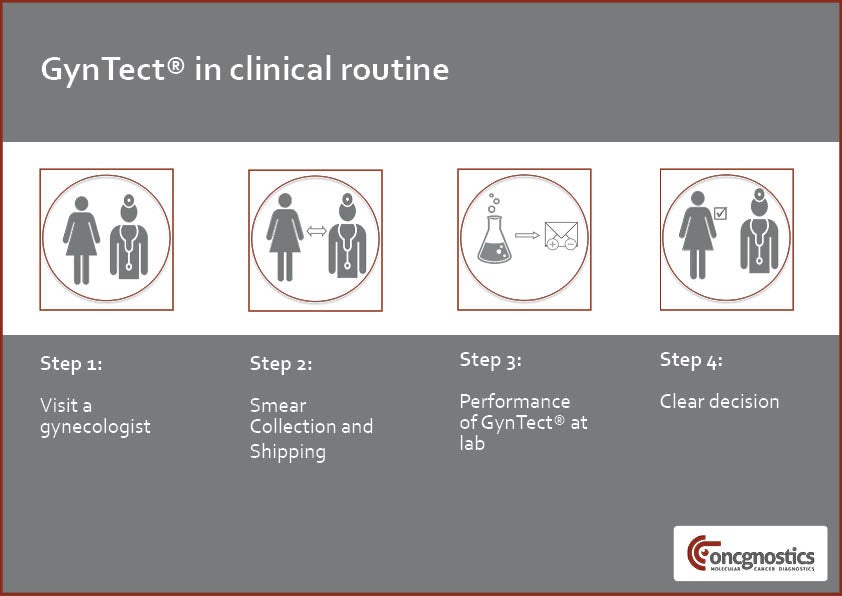
To perform the GynTect test, a gynaecologist takes a smear from the patient’s cervix. This material is transferred to a liquid-based cytology (LBC) vial, which is then sent to a laboratory performing GynTect or directly to Oncgnostics.
The lab performs GynTect in two steps. Initially, any present DNA methylation is fixated by chemical treatment. A methylation-specific (MSP) polymerase chain reaction (PCR) is then used to detect the epigenetic biomarkers.
Cervical smear test to identify methylated genetic material
Abnormal Pap findings frequently lead to surgical removal of the affected tissue. Called conisation, this method may have long-term effects, including increased risk of preterm birth or perinatal death in future pregnancies.
In addition, persistent infection with high-risk HPV may lead to genetic instability. This event may trigger cervical carcinogenesis. Early events in carcinogenesis may be changes within the genetic material such as enhanced methylation of certain cancer-specific DNA regions.
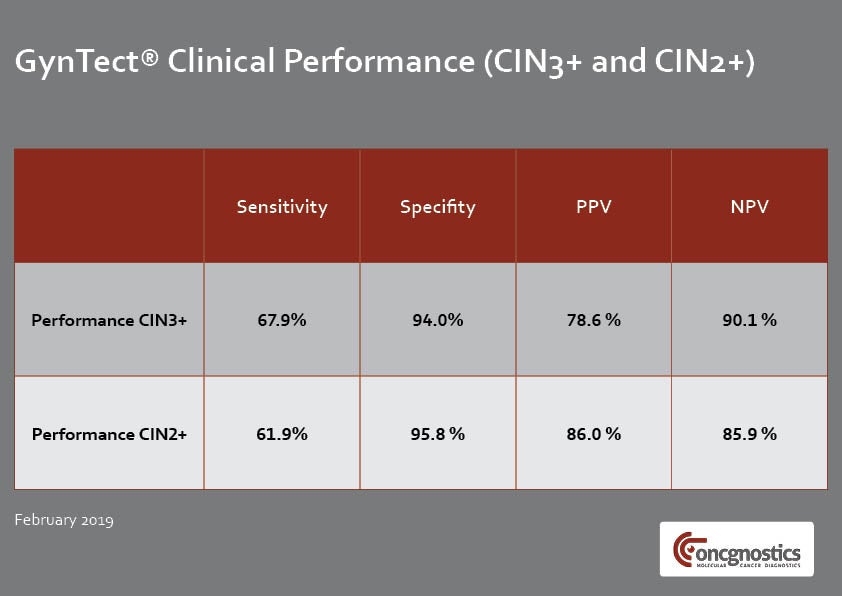
GynTect only requires a single cervical smear in LBC medium and it delivers objective results within a few days. The test detects six regions within the human genetic material that are methylated during cervical carcinogenesis.
Reliable smear test results for gynaecologists
A negative GynTect test result shows that cervical cancer was not present when the testing was performed. If an abnormal Pap smear or an HPV infection was the reason for performing GynTect, these findings can be monitored using the watchful waiting (WAW) approach but further invasive diagnostics are not required.
In the case of a positive GynTect result, a malignant cervical lesion or cancer may be present. Further measures such as colposcopy, biopsy and surgery of the affected tissue are then recommended.
The GynTect test can be performed from the same routine cervical smear material collected in LBC medium for Pap and HPV testing. GynTect’s fast performance leads to timely results and avoids long waiting times.
Development of epigenetic biomarkers for head and neck cancers
Oncgnostics is currently investigating additional applications for its technology, including diagnosis methods for other cancers.
Oncgnostics works in close collaboration with clinical partners such as the Jena University Hospital in Germany. One application is the detection of head and neck cancer. Current diagnostics within this field lead to the late detection of tumours, which are often found when they become macroscopically visible. This late detection reduces the chance of full regression.
Another collaboration aims to help further develop ovarian cancer diagnostics.