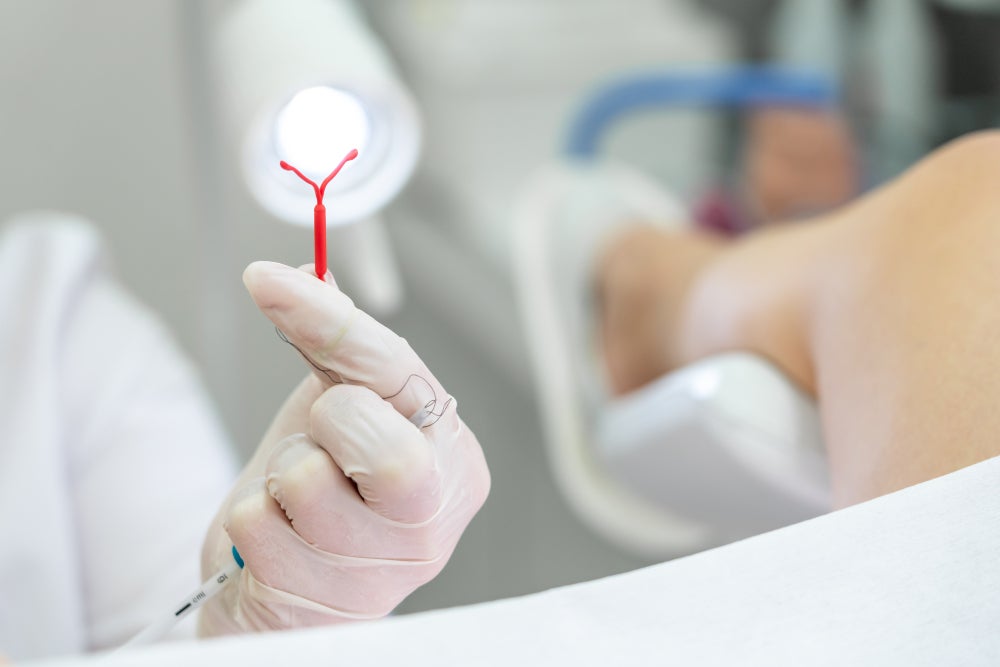
Reproductive health therapies can be central to a woman’s ability to make important decisions about her life. Implantable systems have been a popular vehicle for delivering contraceptive pharmaceuticals for 30 years now. Innovations such as the intrauterine device (IUD) transformed the reproductive healthcare scene, providing an effective, long-term, yet fully reversible method of birth control.
The availability of a range of modern, convenient, and effective contraceptive options is vital. By preventing unintended or high-risk pregnancies, contraception can improve the overall health and wellbeing of women, children, and families. According to the Guttmacher Institute, the unmet need for modern contraceptives is high. Satisfying this need could result in 76,000 fewer maternal deaths and 480,000 fewer newborn deaths every year.[1]
The market for reversible contraceptive devices (including copper and hormonal IUDs, subdermal hormonal implants, and diaphragms) remains strong, with increased government programmes emphasising the benefits and reimbursing their use in order to lower the healthcare spending linked to unplanned pregnancies.
Innovation trends
Although the market for reversible contraceptive devices is well established, innovation continues at a healthy pace. There are currently 45 pipeline reversible contraceptive devices within GlobalData’s database – around half of which are IUDs. Traditional copper IUDs remain widely in use, providing an effective, non-hormonal form of birth control. However, demand is much higher for hormonal IUDs, which use a silicone additive to release progestin over time. Such devices can also help in the alleviation of endometriosis and dysmenorrhea symptoms.
“Over time, copper IUDs have migrated to different polymers, including silicone – the interesting thing there being that you are able to add a drug-eluting profile to them,” says Sean McPherson, senior business development manager, Elkem Silicones. “Some even use copper in combination with a silicone sheath or silicone additive that will elute a drug over time. With a material like silicone, you can begin to add more properties to the device. You start to go from just contraception to maybe contraception plus HIV prevention or STI prophylaxis.”
Silicone has truly opened the doors for novel multipurpose treatment options for women’s reproductive health. “I would think one of the more interesting platforms I’ve seen is the intravaginal ring (IVR),” comments McPherson. “There are multiple companies out there that have designed a platform-style device – a basic silicone ring for support and structure – with the ability to add in drug pods that can target multiple indications.”
The large surface area and rich blood supply of the vaginal area make it a highly effective route for the delivery of contraception and hormone replacement therapy, and the ability for patients to insert and remove IVR devices themselves is another advantage. While multipurpose IVRs combining active pharmaceutical ingredients (APIs) to prevent HIV, HSV, and unplanned pregnancy are currently available, device developers are also working to expand the IVR’s potential to treat a more diverse array of diseases. In the future, a patient-customised IVR, tailored to suit patient-specific indications, would be revolutionary. There is also the potential to leverage microfluidic pump technology to develop a smart, sensorised IVR that releases drugs depending on the patient’s levels.
Design challenges
There are multiple challenges involved in developing implantable drug delivery devices – one being the wide range of body types and chemistries across patients. “Any time you develop any implantable device, you certainly have to take into account that every patient is a little bit different, and so you’re really trying to design for the majority,” says McPherson.
The second big challenge is the APIs, which can become somewhat of a “complex balancing act”, he notes. “There are certainly some different challenges in the chemistries and in the presentations of the APIs themselves,” McPherson explains. “A hormone replacement therapy API, for example, will have a different profile than an HIV preventer such as levonorgestrel. In circumstances where you’d like to deliver two different therapies at the same time, you’re trying to balance two different APIs within one biocompatible material.”
To do so, manufacturers must carefully determine drug loads and elution rates while also making sure no API used has the potential to change the physical properties of the device.
The possibilities of silicone
As a drug-eluting material of choice for years, with wide use in the manufacture of women’s health devices, silicone supports sustained delivery of a range of APIs. Low consistency, lower shore materials such as a 35 to 45 shore liquid silicone rubber (LSR) ensure the patient’s comfort while typically being easier to blend with APIs. But other possibilities, including high consistency rubber (HCR) and adhesives, can also be considered.
“Silicone gives the designer of the device the best chance of success from the get-go due to the array of materials to test,” believes McPherson. Beyond that, he adds, the flexibility within silicone’s various cure kinetics enables manufacturers to find the right system for their specific API/s.
With its high cleanliness, platinum cured is the standard technique for medical-grade materials, however an older technology known as peroxide curing remains acceptable so long as additional steps are taken to eliminate by-products. A tin or titanium catalysed system is a third option with further API possibilities.
Moreover, the best opportunity to optimise the silicone is through the formulations themselves, believes McPherson: “You can change the amount of vinyl in there. You can change the cross-link density without altering the major physical properties, giving designers the chance to dial in the proper elution profile that they’re trying to reach with single or potentially multiple APIs. Overall, there’s quite a bit of flexibility in there if you really work with the silicone manufacturer and have a good knowledge of the end goal.”
Elkem Silicones partners with medical device manufacturers around the world, helping them find the right material to support the design of their drug delivery devices. The company’s medical-grade Silbione™ products meet USP Class VI and ISO 10993 requirements. Within this range, Elkem’s drug delivery silicones have undergone additional testing required for safe, long-term implantation, including the submission of a Drug Master File with the US FDA.
To discover more about the use of silicone materials as modifiable excipients in drug-eluting devices, please download below.
[1] https://www.guttmacher.org/gpr/2017/08/benefits-investing-international-family-planning-and-price-slashing-funding



